Biology:Protein kinase R
Protein kinase RNA-activated also known as protein kinase R (PKR), interferon-induced, double-stranded RNA-activated protein kinase, or eukaryotic translation initiation factor 2-alpha kinase 2 (EIF2AK2) is an enzyme that in humans is encoded by the EIF2AK2 gene on chromosome 2.[1][2] PKR is a serine/tyrosine kinase that is 551 amino acids long.[3]
PKR is inducible by various mechanisms of stress and protects against viral infections.[4] It also has a role in several signaling pathways.[5][6]
Mechanism of action
Protein kinase-R is activated by double-stranded RNA (dsRNA), introduced to the cells by a viral infection.[5] In situations of viral infection, the dsRNA created by viral replication and gene expression binds to the N-terminal domain, activating the protein.[5] PKR activation via dsRNA is length dependent, requiring the dsRNA to be 30 bp in length to bind to PKR molecules.[5] However, excess dsRNA can diminish activation of PKR.[5] Binding to dsRNA is believed to activate PKR by inducing dimerization of the kinase domains and subsequent auto-phosphorylation reactions.[5] PKR can also be activated by the protein PACT via phosphorylation of S287 on its M3 domain.[7] The promoter region of PKR has interferon-stimulated response elements to which Type I interferons (IFN) bind to induce the transcription of PKR genes.[7][8] Some research suggests that PKR can be stimulated by heat shock proteins, heparin, growth factors, bacterial infection, pro-inflammatory cytokines, reactive oxygen species, DNA damage, mechanical stress, and excess nutrient intake.[7]
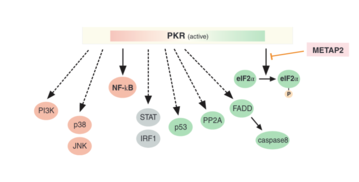
Once active, PKR is able to phosphorylate the eukaryotic translation initiation factor eIF2α.[7] This inhibits further cellular mRNA translation, thereby preventing viral protein synthesis.[6] Overall, this leads to apoptosis of virally infected cells to prevent further viral spread. PKR can also induce apoptosis in bacterial infection by responding to LPS and proinflammatory cytokines.[6] Apoptosis can also occur via PKR activation of the FADD and caspase signaling pathway.[8]
PKR also has pro-inflammatory functions, as it can mediate the activation of the transcription factor NF-kB, by phosphorylating its inhibitory subunit, IkB.[8] This leads to the expression of adhesion molecules and transcription factors that activate them, which induce inflammation responses such as the secretion of pro-inflammatory cytokines.[7] PKR also activates several mitogen-activated protein kinases (MAPK) to lead to inflammation.[8]
To balance the effects of apoptosis and inflammation, PKR has regulatory functions. Active PKR is also able to activate tumor suppressor PP2A which regulates the cell cycle and the metabolism.[9] There is also evidence that PKR is autophagic as a regulatory mechanism.[8]
PKR stress pathway
PKR is in the center of cellular response to different stress signals such as pathogens, lack of nutrients, cytokines, irradiation, mechanical stress, or ER stress.[7] The PKR pathway leads to a stress response through activation of other stress pathways such as JNK, p38, NFkB, PP2A and phosphorylation of eIF2α.[6] ER stress caused by excess of unfolded proteins leads to inflammatory responses.[10] PKR contributes to this response by interacting with several inflammatory kinases such as IKK, JNK, ElF2α, insulin receptors and others.[10] This metabolically activated inflammatory complex is called metabolic inflammasome or metaflammasome.[11][12] Via the JNK signaling pathway, PKR also plays a role in insulin resistance, diabetes, and obesity by phosphorylating IRS1.[13] Inhibiting PKR in mice led to lower inflammation in adipose tissues, increased sensitivity to insulin, and amelioration of diabetic symptoms.[13] PKR also participates in the mitochondrial unfolded protein response (UPRmt).[14] Here, PKR is induced via the transcription factor AP-1 and activated independently of PACT.[14] In this context, PKR has been shown to be relevant to intestinal inflammation.[14]
Viral defense
Viruses have developed many mechanisms to counteract the PKR mechanism. It may be done by Decoy dsRNA, degradation, hiding of viral dsRNA, dimerization block, dephosphorylation of substrate or by a pseudosubstrate.
For instance, Epstein–Barr virus (EBV) uses the gene EBER1 to produce decoy dsRNA. This leads to cancers such as Burkitt's lymphoma, Hodgkin's disease, nasopharyngeal carcinoma and various leukemias.
| Defense type | Virus | Molecule |
|---|---|---|
| Decoy dsRNA | Adenovirus | VAI RNA |
| Epstein–Barr virus | EBER | |
| HIV | TAR | |
| PKR degradation | Poliovirus | 2Apro |
| Hide viral dsRNA | Vaccinia virus | E3L |
| Reovirus | σ3 | |
| Influenza virus | NS1 | |
| Dimerization block | Influenza virus | p58IPK |
| Hepatitis C virus | NS5A | |
| Pseudosubstrate | Vaccinia virus | K3L |
| HIV | Tat | |
| Dephosphorylation of substrate | Herpes simplex virus | ICP34.5 |
Memory and learning
PKR knockout mice or inhibition of PKR in mice enhances memory and learning.[15]
Neuronal degeneration disease
First report in 2002 has been shown that immunohistochemical marker for phosphorylated PKR and eIF2α was displayed positively in degenerating neurons in the hippocampus and the frontal cortex of patients with Alzheimer's disease (AD), suggesting the link between PKR and AD. Additionally, many of these neurons were also immunostained with an antibody for phosphorylated Tau protein.[16] Activated PKR was specifically found in the cytoplasm and nucleus, as well as co-localized with neuronal apoptotic markers.[17] Further studies have assessed the levels of PKR in blood and cerebrospinal fluid (CSF) of AD patients and controls. The result of an analysis of the concentrations of total and phosphorylated PKR (pPKR) in peripheral blood mononuclear cells (PBMCs) in 23 AD patients and 19 control individuals showed statistically significant increased levels of the ratio of phosphorylated PKR/PKR in AD patients compared with controls.[18] Assessments of CSF biomarkers, such as Aβ1-42, Aβ1-40, Tau, and phosphorylated Tau at threonine 181, have been a validated use in clinical research and in routine practice to determine whether patients have CSF abnormalities and AD brain lesions. A study found that "total PKR and pPKR concentrations were elevated in AD and amnestic mild cognitive impairment subjects with a pPKR value (optical density units) discriminating AD patients from control subjects with a sensitivity of 91.1% and a specificity of 94.3%. Among AD patients, total PKR and pPKR levels correlate with CSF p181tau levels. Some AD patients with normal CSF Aß, T-tau, or p181tau levels had abnormal total PKR and pPKR levels".[19] It was concluded that the PKR-eIF2α pro-apoptotic pathway could be involved in neuronal degeneration that leads to various neuropathological lesions as a function of neuronal susceptibility.
PKR and beta amyloid
Activation of PKR can cause accumulation of amyloid β-peptide (Aβ) via de-repression of BACE1 (β-site APP Cleaving Enzyme) expression in Alzheimer Disease patients.[20] Normally, the 5′ untranslated region (5′ UTR) in the BACE1 promoter would fundamentally inhibit the expression of BACE1 gene. However, BACE1 expression can be activated by phosphorylation of eIF2a, which reverses the inhibitory effect exerted by BACE1 5′ UTR. Phosphorylation of eIF2a is triggered by activation of PKR. Viral infection such as herpes simplex virus (HSV) or oxidative stress can both increase BACE1 expression through activation of PKR-eIF2a pathway.[21]
In addition, the increased activity of BACE1 could also lead to β-cleaved carboxy-terminal fragment of β-Amyloid precursor protein (APP-βCTF) induced dysfunction of endosomes in AD.[22] Endosomes are highly active β-Amyloid precursor protein (APP) processing sites, and endosome abnormalities are associated with upregulated expression of early endosomal regulator, Rab5. These are the earliest known disease-specific neuronal response in AD. Increased activity of BACE1 leads to synthesis of the APP-βCTF. An elevated level of βCTF then causes Rab5 overactivation. βCTF recruits APPL1 to rab5 endosomes, where it stabilizes active GTP-Rab5, leading to pathologically accelerated endocytosis, endosome swelling and selectively impaired axonal transport of Rab5 endosomes.
PKR and Tau phosphorylation
It is reported earlier that phosphorylated PKR could co-localize with phosphorylated Tau protein in affected neurons.[23][16] A protein phosphatase-2A inhibitor (PP2A inhibitor) – okadaic acid (OA) – is known to increase tau phosphorylation, Aβ deposition and neuronal death. It is studied that OA also induces PKR phosphorylation and thus, eIF2a phosphorylation. eIF2a phosphorylation then induces activation of transcription factor 4 (ATF4), which induces apoptosis and nuclear translocation, contributing to neuronal death.[24]
Glycogen synthase kinase 3β (GSK-3β) is responsible for tau phosphorylation and controls several cellular functions including apoptosis. Another study demonstrated that tunicamycin or Aβ treatment can induce PKR activation in human neuroblastoma cells and can trigger GSK3β activation, as well as tau phosphorylation. They found that in AD brains, both activated PKR and GSK3β co-localize with phosphorylated tau in neurons. In SH-SY5Y cell cultures, tunicamycin and Aβ(1-42) activate PKR, which then can modulate GSK-3β activation and induce tau phosphorylation, apoptosis. All these processes are attenuated by PKR inhibitors or PKR siRNA. PKR could represent a crucial signaling point relaying stress signals to neuronal pathways by interacting with transcription factor or indirectly controlling GSK3β activation, leading to cellular degeneration in AD.[25]
Fetal alcohol syndrome
PKR also mediates ethanol-induced protein synthesis inhibition and apoptosis which is linked to fetal alcohol syndrome.[26]
Interactions
Protein kinase R has been shown to interact with:
- ASK1,[27]
- DNAJC3,[28]
- ILF3,[29][30][31][32]
- METAP2,[33]
- P53,[34]
- PPP1CA,[35]
- PRKRA,[36][37]
- STAT1,[38][39] and
- TARBP2.[40][41]
References
- ↑ "Entrez Gene: EIF2AK2 eukaryotic translation initiation factor 2-alpha kinase 2". https://www.ncbi.nlm.nih.gov/sites/entrez?db=gene&cmd=retrieve&dopt=default&list_uids=5610&rn=1.
- ↑ "Identification of double-stranded RNA-binding domains in the interferon-induced double-stranded RNA-activated p68 kinase". Proceedings of the National Academy of Sciences of the United States of America 89 (12): 5447–5451. June 1992. doi:10.1073/pnas.89.12.5447. PMID 1351683. Bibcode: 1992PNAS...89.5447F.
- ↑ "Emerging role of protein kinases in diabetes mellitus: From mechanism to therapy". Advances in Protein Chemistry and Structural Biology. Protein Kinases in Drug Discovery (Academic Press) 124: 47–85. January 2021. doi:10.1016/bs.apcsb.2020.11.001. ISBN 9780323853132. PMID 33632470.
- ↑ "The search for a PKR code-differential regulation of protein kinase R activity by diverse RNA and protein regulators". RNA 25 (5): 539–556. May 2019. doi:10.1261/rna.070169.118. PMID 30770398.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 "The Role of Nucleic Acid Sensing in Controlling Microbial and Autoimmune Disorders". International Review of Cell and Molecular Biology. Nucleic Acid Sensing and Immunity - Part B (Academic Press) 345: 35–136. 2019-01-01. doi:10.1016/bs.ircmb.2018.08.002. ISBN 9780128159811. PMID 30904196.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 "Protein kinase R and its cellular regulators in cancer: An active player or a surveillant?". Wiley Interdisciplinary Reviews. RNA 11 (2): e1558. March 2020. doi:10.1002/wrna.1558. PMID 31231984. https://onlinelibrary.wiley.com/doi/10.1002/wrna.1558.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 "PKR: A Kinase to Remember". Frontiers in Molecular Neuroscience 11: 480. 2019. doi:10.3389/fnmol.2018.00480. PMID 30686999.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 "Protein Kinase R in Bacterial Infections: Friend or Foe?". Frontiers in Immunology 12: 702142. 2021. doi:10.3389/fimmu.2021.702142. PMID 34305942.
- ↑ "PKR inhibits the DNA damage response, and is associated with poor survival in AML and accelerated leukemia in NHD13 mice". Blood 126 (13): 1585–1594. September 2015. doi:10.1182/blood-2015-03-635227. PMID 26202421.
- ↑ Jump up to: 10.0 10.1 "The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis". FEBS Letters 581 (22): 4325–4332. September 2007. doi:10.1016/j.febslet.2007.08.001. PMID 17716668.
- ↑ "Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action". Microbiology and Molecular Biology Reviews 70 (4): 1032–1060. December 2006. doi:10.1128/MMBR.00027-06. PMID 17158706.
- ↑ "Endoplasmic reticulum stress and the inflammatory basis of metabolic disease". Cell 140 (6): 900–917. March 2010. doi:10.1016/j.cell.2010.02.034. PMID 20303879.
- ↑ Jump up to: 13.0 13.1 Endogenous Retroelements and the Host Innate Immune Sensors. Advances in Immunology. 132. Academic Press. 2016-01-01. pp. 47–69. doi:10.1016/bs.ai.2016.07.001. ISBN 9780128047972.
- ↑ Jump up to: 14.0 14.1 14.2 "Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation". Gut 61 (9): 1269–1278. September 2012. doi:10.1136/gutjnl-2011-300767. PMID 21997551.
- ↑ "Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition". Cell 147 (6): 1384–1396. December 2011. doi:10.1016/j.cell.2011.11.029. PMID 22153080.
- ↑ Jump up to: 16.0 16.1 "Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease". NeuroReport 13 (18): 2429–2432. December 2002. doi:10.1097/00001756-200212200-00011. PMID 12499843.
- ↑ "Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer's disease". Neuroscience 139 (4): 1343–1354. 2006. doi:10.1016/j.neuroscience.2006.01.047. PMID 16581193.
- ↑ "Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer's disease". Dementia and Geriatric Cognitive Disorders 22 (4): 320–326. 2006. doi:10.1159/000095562. PMID 16954686.
- ↑ "Increased cerebrospinal fluid levels of double-stranded RNA-dependant protein kinase in Alzheimer's disease". Biological Psychiatry 71 (9): 829–835. May 2012. doi:10.1016/j.biopsych.2011.11.031. PMID 22281122.
- ↑ "Activation of PKR causes amyloid ß-peptide accumulation via de-repression of BACE1 expression". PLOS ONE 6 (6): e21456. 2011-06-28. doi:10.1371/journal.pone.0021456. PMID 21738672. Bibcode: 2011PLoSO...621456I.
- ↑ "Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2α pathway". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1822 (6): 885–896. June 2012. doi:10.1016/j.bbadis.2012.01.009. PMID 22306812.
- ↑ "Evidence that the rab5 effector APPL1 mediates APP-βCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer's disease". Molecular Psychiatry 21 (5): 707–716. May 2016. doi:10.1038/mp.2015.97. PMID 26194181.
- ↑ "Activation of the cell stress kinase PKR in Alzheimer's disease and human amyloid precursor protein transgenic mice". Neurobiology of Disease 14 (1): 52–62. October 2003. doi:10.1016/S0969-9961(03)00086-X. PMID 13678666.
- ↑ "Activation of eukaryotic initiation factor-2 α-kinases in okadaic acid-treated neurons". Neuroscience 169 (4): 1831–1839. September 2010. doi:10.1016/j.neuroscience.2010.06.016. PMID 20600673.
- ↑ "Modulation of tau phosphorylation by the kinase PKR: implications in Alzheimer's disease". Brain Pathology 21 (2): 189–200. March 2011. doi:10.1111/j.1750-3639.2010.00437.x. PMID 21029237.
- ↑ "Interaction between RAX and PKR modulates the effect of ethanol on protein synthesis and survival of neurons". The Journal of Biological Chemistry 281 (23): 15909–15915. June 2006. doi:10.1074/jbc.M600612200. PMID 16574643.
- ↑ "Double-stranded RNA-activated protein kinase interacts with apoptosis signal-regulating kinase 1. Implications for apoptosis signaling pathways". European Journal of Biochemistry 269 (24): 6126–6132. December 2002. doi:10.1046/j.1432-1033.2002.03325.x. PMID 12473108.
- ↑ "The P58 cellular inhibitor complexes with the interferon-induced, double-stranded RNA-dependent protein kinase, PKR, to regulate its autophosphorylation and activity". The Journal of Biological Chemistry 271 (3): 1702–1707. January 1996. doi:10.1074/jbc.271.3.1702. PMID 8576172.
- ↑ "Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR". The Journal of Biological Chemistry 276 (34): 32300–32312. August 2001. doi:10.1074/jbc.M104207200. PMID 11438536.
- ↑ "Nuclear factor-90 of activated T-cells: A double-stranded RNA-binding protein and substrate for the double-stranded RNA-dependent protein kinase, PKR". Biochemistry 38 (19): 6361–6368. May 1999. doi:10.1021/bi982410u. PMID 10320367.
- ↑ "Nuclear factor 90 is a substrate and regulator of the eukaryotic initiation factor 2 kinase double-stranded RNA-activated protein kinase". The Journal of Biological Chemistry 276 (35): 32522–32530. August 2001. doi:10.1074/jbc.M104408200. PMID 11438540.
- ↑ "DRBP76, a double-stranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR". The Journal of Biological Chemistry 274 (29): 20432–20437. July 1999. doi:10.1074/jbc.274.29.20432. PMID 10400669.
- ↑ "In vivo regulation of the dsRNA-dependent protein kinase PKR by the cellular glycoprotein p67". Biochemistry 39 (51): 16016–16025. December 2000. doi:10.1021/bi001754t. PMID 11123929.
- ↑ "The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro". Oncogene 18 (17): 2690–2702. April 1999. doi:10.1038/sj.onc.1202620. PMID 10348343.
- ↑ "The direct binding of the catalytic subunit of protein phosphatase 1 to the PKR protein kinase is necessary but not sufficient for inactivation and disruption of enzyme dimer formation". The Journal of Biological Chemistry 277 (39): 36109–36117. September 2002. doi:10.1074/jbc.M205109200. PMID 12138106.
- ↑ "The C-terminal, third conserved motif of the protein activator PACT plays an essential role in the activation of double-stranded-RNA-dependent protein kinase (PKR)". The Biochemical Journal 366 (Pt 1): 175–186. August 2002. doi:10.1042/BJ20020204. PMID 11985496.
- ↑ "PACT, a protein activator of the interferon-induced protein kinase, PKR". The EMBO Journal 17 (15): 4379–4390. August 1998. doi:10.1093/emboj/17.15.4379. PMID 9687506.
- ↑ "Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways". The EMBO Journal 16 (6): 1291–1304. March 1997. doi:10.1093/emboj/16.6.1291. PMID 9135145.
- ↑ "Enhanced antiviral and antiproliferative properties of a STAT1 mutant unable to interact with the protein kinase PKR". The Journal of Biological Chemistry 276 (17): 13727–13737. April 2001. doi:10.1074/jbc.M011240200. PMID 11278865.
- ↑ "Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo". Proceedings of the National Academy of Sciences of the United States of America 92 (21): 9445–9449. October 1995. doi:10.1073/pnas.92.21.9445. PMID 7568151. Bibcode: 1995PNAS...92.9445C.
- ↑ "Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression". The Journal of Biological Chemistry 276 (36): 33899–33905. September 2001. doi:10.1074/jbc.M103584200. PMID 11438532.
Further reading
- "PKR; a sentinel kinase for cellular stress". Oncogene 18 (45): 6112–6120. November 1999. doi:10.1038/sj.onc.1203127. PMID 10557102.
- "The dsRNA protein kinase PKR: virus and cell control". Biochimie 89 (6–7): 799–811. 2007. doi:10.1016/j.biochi.2007.03.001. PMID 17451862.
- "Mechanism of interferon action: cDNA structure, expression, and regulation of the interferon-induced, RNA-dependent P1/eIF-2 alpha protein kinase from human cells". Virology 188 (1): 33–46. May 1992. doi:10.1016/0042-6822(92)90732-5. PMID 1373553.
- "Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase". Virology 188 (1): 47–56. May 1992. doi:10.1016/0042-6822(92)90733-6. PMID 1373554.
- "A synthetic peptide substrate for initiation factor-2 kinases". Biochemical and Biophysical Research Communications 178 (2): 430–437. July 1991. doi:10.1016/0006-291X(91)90125-Q. PMID 1677563.
- "Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon". Cell 62 (2): 379–390. July 1990. doi:10.1016/0092-8674(90)90374-N. PMID 1695551.
- "Translational regulation by HIV leader RNA, TAT, and interferon-inducible enzymes". Journal of Experimental Pathology 5 (2): 69–77. 1991. PMID 1708818.
- "Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product". Science 247 (4947): 1216–1219. March 1990. doi:10.1126/science.2180064. PMID 2180064. Bibcode: 1990Sci...247.1216R.
- "HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR". Virology 213 (2): 413–424. November 1995. doi:10.1006/viro.1995.0014. PMID 7491766.
- "Chromosomal assignment of the interferon-inducible double-stranded RNA-dependent protein kinase (PRKR) to human chromosome 2p21-p22 and mouse chromosome 17 E2". Genomics 16 (3): 765–767. June 1993. doi:10.1006/geno.1993.1262. PMID 7686883.
- "Localization of the human interferon-induced, ds-RNA activated p68 kinase gene (PRKR) to chromosome 2p21-p22". Genomics 16 (3): 768–770. June 1993. doi:10.1006/geno.1993.1263. PMID 7686884.
- "A 68-kDa kinase and NADPH oxidase component p67phox are targets for Cdc42Hs and Rac1 in neutrophils". The Journal of Biological Chemistry 270 (18): 10717–10722. May 1995. doi:10.1074/jbc.270.18.10717. PMID 7738010.
- "Translational regulation by the interferon-induced double-stranded-RNA-activated 68-kDa protein kinase". Proceedings of the National Academy of Sciences of the United States of America 90 (10): 4621–4625. May 1993. doi:10.1073/pnas.90.10.4621. PMID 8099444. Bibcode: 1993PNAS...90.4621B.
- "Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity". Cell 84 (6): 853–862. March 1996. doi:10.1016/S0092-8674(00)81064-8. PMID 8601309.
- "Structural organization of the human gene (PKR) encoding an interferon-inducible RNA-dependent protein kinase (PKR) and differences from its mouse homolog". Genomics 36 (1): 197–201. August 1996. doi:10.1006/geno.1996.0446. PMID 8812437.
- "Autophosphorylation sites participate in the activation of the double-stranded-RNA-activated protein kinase PKR". Molecular and Cellular Biology 16 (11): 6295–6302. November 1996. doi:10.1128/mcb.16.11.6295. PMID 8887659.
- "Mechanism of interferon action sequence of the human interferon-inducible RNA-dependent protein kinase (PKR) deduced from genomic clones". Gene 178 (1–2): 191–193. October 1996. doi:10.1016/0378-1119(96)00314-9. PMID 8921913.
 |