Biology:Flavodoxin
Flavodoxins (Fld) are small, soluble electron-transfer proteins.[1][2] Flavodoxins contains flavin mononucleotide as prosthetic group. The structure of flavodoxin is characterized by a five-stranded parallel beta sheet, surrounded by five alpha helices.[3] They have been isolated from prokaryotes, cyanobacteria, and some eukaryotic algae.[2]
Background
Originally found in cyanobacteria and clostridia, flavodoxins were discovered over 50 years ago.[4] These proteins evolved from an anaerobic environment, due to selective pressures. Ferredoxin, another redox protein, was the only protein able to be used in this manner. However, when oxygen became present in the environment, iron became limited. Ferredoxin is iron-dependant as well as oxidant-sensitive. Under these limited iron conditions, ferredoxin was no longer preferred. Flavodoxin on the other hand is the opposite of these traits, as it is oxidant-resistant and has iron-free isofunctional counterparts. Therefore, for some time flavodoxin was the primary redox protein. Now however, when ferredoxin and flavodoxin are present in the same genome, ferredoxin is still used but under low iron conditions, flavodoxin is induced.[5]
Structure
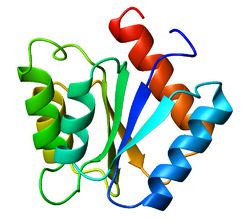
Three forms of flavodoxin exist: Oxidized, (OX) semiquinone, (SQ) and hydroquinone (HQ). While relatively small (Mw = 15-22 kDa),[6] flavodoxins exist in "long" and "short" chain classifications. Short chain flavodoxins contain between 140 and 180 amino acid residues,[4] while long chain flavodoxins include a 20 amino acid insertion into the last beta-strand. These residues form a loop which may be used to increase the binding affinity of flavin mononucleotide as well as assist in the formation of folded intermediates. However, it is still not certain what the loops true function is. In addition, the flavin mononucleotide is non-covalently bound to the flavodoxin protein and works to shuttle electrons.[4][5]
Medical applications
Heliobacter pylori (Hp), the most prevalent human gastric pathogen, requires flavodoxins in its essential POR (pyruvate oxidoreductase enzyme complex) [7] used in pyruvate decarboxylation. Most flavodoxins have a large hydrophobic residue such as tryptophan near the FMN, but Hp has an alanine residue instead, allowing for a pocket of solute to form. Current research is being done to identify non toxic, Hp specific flavodoxin inhibitors for the purpose of treating infection.[8]
Mechanism
Flavodoxins require a highly negative redox potential to be active. The semiquinone conformation is stabilized by a hydrogen bond to the N-5 position of the flavin. This bond, as well as a common tryptophan residue near the binding site, aid in lowering SQ reactivity. The hydroquinone form is forced into a planar conformation, destabilizing it.[9] Electron transfer occurs at the dimethylbenzene ring of the FMN.
Flavodoxins in Cyanobacteria
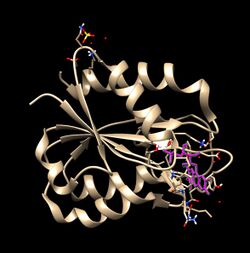
In cyanobacteria such as Nostoc sp., flavodoxins are heterocyst-specific,[10] and used in photosystem 1 to deliver electrons to nitrogenase, as well as reducing N2 and NADP+, nitrogen fixation and H2 formation.[6]
References
- ↑ "Flavodoxins: sequence, folding, binding, function and beyond". Cellular and Molecular Life Sciences 63 (7–8): 855–864. April 2006. doi:10.1007/s00018-005-5514-4. PMID 16465441.
- ↑ 2.0 2.1 "The long goodbye: the rise and fall of flavodoxin during plant evolution". Journal of Experimental Botany 65 (18): 5161–5178. October 2014. doi:10.1093/jxb/eru273. PMID 25009172.
- ↑ "Crystal structure of oxidized flavodoxin, an essential protein in Helicobacter pylori". Protein Science 11 (2): 253–261. February 2002. doi:10.1110/ps.28602. PMID 11790835.
- ↑ 4.0 4.1 4.2 "Structure and function of an unusual flavodoxin from the domain Archaea". Proceedings of the National Academy of Sciences of the United States of America 116 (51): 25917–25922. December 2019. doi:10.1073/pnas.1908578116. PMID 31801875. Bibcode: 2019PNAS..11625917P.
- ↑ 5.0 5.1 "Folding of proteins with a flavodoxin-like architecture". The FEBS Journal 284 (19): 3145–3167. October 2017. doi:10.1111/febs.14077. PMID 28380286.
- ↑ 6.0 6.1 "The importance of flavodoxin for environmental stress tolerance in photosynthetic microorganisms and transgenic plants. Mechanism, evolution and biotechnological potential". FEBS Letters 586 (18): 2917–2924. August 2012. doi:10.1016/j.febslet.2012.07.026. PMID 22819831.
- ↑ "Towards a new therapeutic target: Helicobacter pylori flavodoxin". Biophysical Chemistry 115 (2–3): 267–276. April 2005. doi:10.1016/j.bpc.2004.12.045. PMID 15752617.
- ↑ "Flavodoxins as Novel Therapeutic Targets against Helicobacter pylori and Other Gastric Pathogens". International Journal of Molecular Sciences 21 (5): 1881. March 2020. doi:10.3390/ijms21051881. PMID 32164177.
- ↑ "Structure-function relations in flavodoxins". Molecular and Cellular Biochemistry 33 (1–2): 13–24. December 1980. doi:10.1007/BF00224568. PMID 6782445.
- ↑ "Gas exchange in the filamentous cyanobacterium Nostoc punctiforme strain ATCC 29133 and Its hydrogenase-deficient mutant strain NHM5". Applied and Environmental Microbiology 70 (4): 2137–2145. April 2004. doi:10.1128/AEM.70.4.2137-2145.2004. PMID 15066806. Bibcode: 2004ApEnM..70.2137L.
External links
- Flavodoxin at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Flavodoxin Folding and Stability Research at Wageningen University, the Netherlands"
- "The crossovers of flavodoxin" at virginia.edu
- Diagram at ohio-state.edu
 |
