Chemistry:2-C-Methyl-D-erythritol-2,4-cyclopyrophosphate
| |
| |
| Names | |
|---|---|
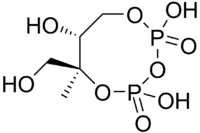
| Systematic IUPAC name
(6S,7R)-2,4,7-Trihydroxy-6-(hydroxymethyl)-6-methyl-1,3,5,2λ5,4λ5-trioxadiphosphocane-2,4-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| MeSH | 2-methyl-butan-1,2,3,4-tetraol-2,4-cyclopyrophosphate |
PubChem CID
|
|
| |
| Properties | |
| C5H12O9P2 | |
| Molar mass | 278.09 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-C-Methyl-d-erythritol-2,4-cyclopyrophosphate (MEcPP) (also 2-C-Methyl-d-erythritol-2,4-cyclodiphosphate) is an intermediate in the MEP pathway (non-mevalonate) of isoprenoid precursor biosynthesis.[1] MEcPP is produced by MEcPP synthase (IspF) and is a substrate for HMB-PP synthase (IspG).
Under conditions of oxidative stress, MEcPP accumulates in certain bacteria.[2] MEcPP releases histone-like proteins from DNA, triggering nucleoid decondensation in Chlamydia trachomatis during the process of terminal differentiation.[3] Abiotic stresses to plants, including wounding and excessive high-light exposure, lead to an increase in MEcPP accumulation in chloroplasts. Transported from the chloroplast to the plant cell nucleus, MEcPP engages in retrograde signalling that leads to the specific induction of nuclear-encoded stress-response genes.[4]
References
- ↑ "Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-D-erythritol 2-phosphate to 2C-methyl-D-erythritol 2,4-cyclodiphosphate". Proc. Natl. Acad. Sci. USA 97 (6): 2486–90. 2000. doi:10.1073/pnas.040554697. PMID 10694574. Bibcode: 2000PNAS...97.2486H.
- ↑ "Bacterial oxidative-stress substance is 2-C-methyl-D-erythritol 2,4-cyclopyrophosphate". Biochem J 295 (3): 901–2. 1993. doi:10.1042/bj2950901. PMID 8240308.
- ↑ "Chlamydial histone-DNA interactions are disrupted by a metabolite in the methylerythritol phosphate pathway of isoprenoid biosynthesis". Proc. Natl. Acad. Sci. USA 101 (19): 7451–6. 2004. doi:10.1073/pnas.0400754101. PMID 15123794.
- ↑ "Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes". Cell 149 (7): 1525–35. 2012. doi:10.1016/j.cell.2012.04.038. PMID 22726439.
 |

