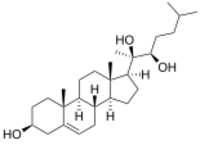
Chemistry:20α,22R-Dihydroxycholesterol
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
(22R)-Cholest-5-ene-3β,20,22-triol
| |
| Systematic IUPAC name
(2R,3R)-2-[(1S,3aS,3bS,7S,9aR,9bS,11aS)-7-Hydroxy-9a,11a-dimethyl-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-1-yl]-6-methylheptane-2,3-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| 2339391 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C27H46O3 | |
| Molar mass | 418.652 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
20α,22R-Dihydroxycholesterol, or (3β)-cholest-5-ene-3,20,22-triol is an endogenous, metabolic intermediate in the biosynthesis of the steroid hormones from cholesterol.[1][2] Cholesterol ((3β)-cholest-5-en-3-ol) is hydroxylated by cholesterol side-chain cleavage enzyme (P450scc) to form 22R-hydroxycholesterol, which is subsequently hydroxylated again by P450scc to form 20α,22R-dihydroxycholesterol, and finally the bond between carbons 20 and 22 is cleaved by P450scc to form pregnenolone ((3β)-3-hydroxypregn-5-en-20-one),[1][2] the precursor to the steroid hormones.
See also
References
- ↑ 1.0 1.1 "Biosynthesis of pregnenolone from 22-hydroxycholesterol". The Journal of Biological Chemistry 237 (3): 703–4. March 1962. doi:10.1016/S0021-9258(18)60359-X. PMID 13878470.
- ↑ 2.0 2.1 "The catalytic cycle of cytochrome P-450scc and intermediates in the conversion of cholesterol to pregnenolone". European Journal of Biochemistry 140 (3): 583–91. May 1984. doi:10.1111/j.1432-1033.1984.tb08142.x. PMID 6723652.
 |
