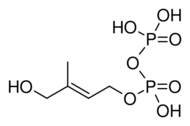
Chemistry:(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate
| |
| |
| Names | |
|---|---|
| IUPAC name
(E)-4-hydroxy-3-methylbut-2-enoxy-oxidophosphoryl phosphate
| |
| Other names
(E)-4-hydroxy-dimethylallyl pyrophosphate
HDMAPP (E)-4-Hydroxy-3-methyl-but-2-enyl diphosphate HMBDP | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C5H12O8P2 | |
| Molar mass | 262.091 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP or HMB-PP) is an intermediate of the MEP pathway (non-mevalonate pathway) of isoprenoid biosynthesis.[1][2] The enzyme HMB-PP synthase (GcpE, IspG) catalyzes the conversion of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) into HMB-PP. HMB-PP is then converted further to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH).
HMB-PP is an essential metabolite in most pathogenic bacteria including Mycobacterium tuberculosis as well as in malaria parasites, but is absent from the human host.[3]
HMB-PP is the physiological activator ("phosphoantigen") for human Vγ9/Vδ2 T cells, the major γδ T cell population in peripheral blood. With a bioactivity of 0.1 nM it is 10,000-10,000,000 times more potent than any other natural compound, such as IPP or alkyl amines. HMB-PP functions in this capacity by binding the B30.2 domain of BTN3A1.[4]
References
- ↑ Rohmer, M; Rohmer, Michel (1999). "The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants". Natural Product Reports 16 (5): 565–74. doi:10.1039/a709175c. PMID 10584331.
- ↑ Fox, DT; Poulter, CD (2002). "Synthesis of (E)-4-hydroxydimethylallyl diphosphate. An intermediate in the methyl erythritol phosphate branch of the isoprenoid pathway". The Journal of Organic Chemistry 67 (14): 5009–10. doi:10.1021/jo0258453. PMID 12098326.
- ↑ Eisenreich, W; Bacher, A; Arigoni, D; Rohdich, F (2004). "Biosynthesis of isoprenoids via the non-mevalonate pathway". Cellular and Molecular Life Sciences 61 (12): 1401–26. doi:10.1007/s00018-004-3381-z. PMID 15197467.
- ↑ "Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor Periplakin". J Immunol 194 (5): 2390–8. 2015. doi:10.4049/jimmunol.1401064. PMID 25637025.
External links
- 4-hydroxy-3-methylbut-2-enyl+pyrophosphate at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

