Chemistry:Allylbenzene
From HandWiki
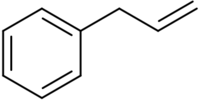
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Prop-2-enyl)benzene[1] | |
| Other names
3-Phenyl-1-propene; 2-Propenylbenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10 | |
| Molar mass | 118.179 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.893 g/cm3 |
| Melting point | −40 °C (−40 °F; 233 K) |
| Boiling point | 156 °C (313 °F; 429 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Allylbenzene or 3-phenylpropene is an organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to an allyl group. Allylbenzene isomerizes to trans-propenylbenzene.[2]
In plant biochemistry, the allylbenzene skeleton is the parent (simplest representation) of many phenylpropanoids. Prominent allylbenzenes include eugenol, safrole, and many others.[3]
References
- ↑ "Allylbenzene". https://pubchem.ncbi.nlm.nih.gov/compound/9309#section=IUPAC-Name&fullscreen=true.
- ↑ Hassam, Mohammad; Taher, Abu; Arnott, Gareth E.; Green, Ivan R.; van Otterlo, Willem A. L. (2015). "Isomerization of Allylbenzenes". Chemical Reviews 115 (11): 5462–5569. doi:10.1021/acs.chemrev.5b00052. PMID 25993416.
- ↑ Vogt, Thomas (2010). "Phenylpropanoid Biosynthesis". Molecular Plant 3 (1): 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
External links
 |
