Chemistry:G-130
From HandWiki
Short description: Chemical compound
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
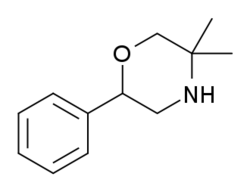
| Formula | C12H17NO |
| Molar mass | 191.274 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
G-130 (GP-130, 2-Phenyl-5,5-dimethyltetrahydro-1,4-oxazine)[1] is a drug with stimulant and anorectic effects, related to phenmetrazine.[2][3][4]
Structural analogs
Compounds related to G-130 and radafaxine were synthesized that behave as combined inhibitors of monoamine uptake and nicotinic acetylcholine receptors.[5]
Synthesis
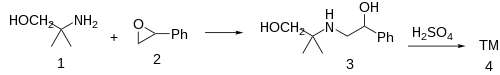
Ex 1: 2 moles of 2-methyl-2-aminopropanol (aminomethyl propanol) (1) is reacted with 1 moles of styrene oxide (phenyloxirane) [96-09-3] (2) in 0.2 mole water.
Ex 2: Fepradinol [36981-91-6] [63075-47-8] (3) is treated with acid, to cyclize to the morpholine ring.
See also
- 2-Phenyl-3,6-dimethylmorpholine
- 3-Fluorophenmetrazine
- 4-Methylphenmetrazine
- Phendimetrazine
- Manifaxine
References
- ↑ 1.0 1.1 "Diethanolamine derivatives" GB patent 1336732, published 1973-11-07, assigned to Instituto Gentili SPA
- ↑ "Pharmacological and toxicological study of a new psychotropic stimulant: the 2-phenyl-5-dimethyl-tetrahydro-1,4-oxazine, in comparison with d,l-amphetamine, phenmetrazine and pemoline-Mg". Arzneimittel-Forschung 23 (6): 810–816. June 1973. PMID 4740767.
- ↑ "Toxicological and teratological study of 2-phenyl-5,5-dimethyl-tetrahydro-1,4-oxazine hydrochloride (G 130), a psychostimulant drug". Arzneimittel-Forschung 24 (10): 1627–1632. October 1974. PMID 4479774.
- ↑ "Antagonism of psychostimulant 2-phenyl-5,5-dimethyl-tetrahydro-1,4-oxazine hydrochloride (G 130) to central nervous system depressing drugs. Monoamine oxidase inhibitory activity and norepinephrine and serotonin induced changes. Comparison with dL-amphetamine". Arzneimittel-Forschung 24 (12): 2025–2029. December 1974. PMID 4480283.
- ↑ "Synthesis of 2-(substituted phenyl)-3,5,5-trimethylmorpholine analogues and their effects on monoamine uptake, nicotinic acetylcholine receptor function, and behavioral effects of nicotine". Journal of Medicinal Chemistry 54 (5): 1441–1448. March 2011. doi:10.1021/jm1014555. PMID 21319801.
- ↑ ES508287 (application number) idem ES patent 8300676 (1983 to Elmu Sa).
 |
