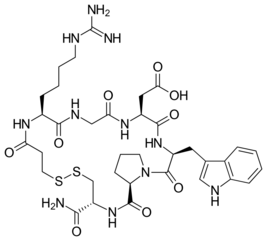
Chemistry:Arginylglycylaspartic acid

| |
| Names | |
|---|---|
| Systematic IUPAC name
(2S)-2-[[2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]acetyl]amino]butanedioic acid | |
| Other names
L-Arginyl-Glycyl-L-Aspartic acid; Arg-Gly-Asp
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | RGD Peptide[citation needed] |
| ChEMBL | |
| ChemSpider | |
| MeSH | arginyl-glycyl-aspartic+acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H22N6O6 | |
| Molar mass | 346.344 g·mol−1 |
| log P | −3.016 |
| Acidity (pKa) | 2.851 |
| Basicity (pKb) | 11.146 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
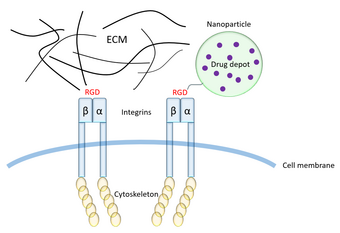
Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from Drosophila to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins.[1] The discovery of RGD and elucidation of how RGD binds to integrins has led to the development of a number of drugs and diagnostics,[2] while the peptide itself is used ubiquitously in bioengineering.[3] Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion.
Discovery
RGD was identified as the minimal recognition sequence within fibronectin required for cell attachment by Ruoslahti and Pierschbacher in the early 1980s. To do this, the authors synthesized various peptides based on the hypothesized cell attachment site of fibronectin. They then coupled those peptides to protein-coated plastic and tested each for cell attachment-promoting activity. Only those that contained the RGD sequence were found to enhance cell attachment. Further, they showed that peptides containing RGD were able to inhibit cell attachment to fibronectin-coated substrates, whereas peptides not containing RGD did not.[4]
These foundational studies also identified the cellular receptors that recognize the sequence. These studies utilized a synthetic RGD-containing peptide to isolate the putative receptors, and then demonstrated that liposomes containing the isolated proteins could bind to fibronectin, in much the same way as cells with surface receptors.[5][6] The discovered receptors were later named integrins.[7][8] The RGD motif is presented in slightly different ways in different proteins, making it possible for the many RGD-binding integrins to selectively distinguish individual adhesion proteins.[9][10]
Use in drug discovery
Understanding of the molecular basis of binding to integrins has enabled the development of several drugs for cardiovascular disease and cancer, including eptifibatide, tirofiban and cilengitide.[11][2] These drugs inhibit integrin binding. PET radiotracers such as fluciclatide utilize RGD-containing peptides to home to tumors, allowing for cancer monitoring.[12]
Cardiovascular disease
Eptifibatide and tirofiban are anti-clotting drugs indicated to prevent thrombosis in acute ischemic coronary syndromes.[13][14] Eptifibatide is additionally FDA approved for patients undergoing percutaneous coronary intervention.[15] These drugs block activation of the integrin responsible for aggregation of platelets (αIIbβ3, also known as glycoprotein IIb/IIIa) in response to the blood glycoproteins fibrinogen and von Willebrand factor. Eptifibatide (marketed as Integrilin) is a cyclic (circular) seven amino acid peptide, whereas tirofiban is a small molecule designed to mimic the chemistry and binding affinity of the RGD sequence.[16][17]
Cancer
Cilengitide, a cyclic pentapeptide (RGDfV), is an investigational drug intended to block the growth of new blood vessels in tumors by interfering with the activation of integrin αVβ3.[18] This integrin is upregulated in tumor and activated endothelial cells.[19] This and other anti-angiogenic therapies depend on cutting off the blood supply to the tumor micro-environment, leading to hypoxia and necrosis.[20] Cilengitide has been evaluated for the treatment of glioblastoma, but, as is the case for other anti-angiogenic therapies, has not been shown to alter progression or improve survival either alone or in combination with standard treatments.[21]
CEND-1, also known as iRGD, is a cyclic peptide that homes to tumors via binding to integrin alpha V receptors.[22] It also binds and activates neuropilin-1, leading to a temporary opening of the tumor and an enhanced delivery of anti-cancer agents into the tumor tissue. It is currently being tested in clinical trials in solid tumor patients.[23]
Diagnostics
As anti-angiogenic cancer therapies have achieved widespread use, there has been increased interest in non-invasive monitoring of angiogenesis. One of the most extensively examined targets of angiogenesis is integrin αVβ3. Radiolabeled peptides containing RGD show high affinity and selectivity for integrin αVβ3 and are being investigated as tools to monitor treatment response of tumors via PET imaging.[24] These include 18F-Galacto-RGD, 18F-Fluciclatide-RGD, 18F-RGD-K5, 68Ga-NOTA-RGD, 68Ga-NOTA-PRGD2, 18F-Alfatide, 18F-Alfatide II, and 18F-FPPRGD2.[19][12][24] In a meta-analysis of studies using PET/CT in patients with cancer, it was shown that this diagnostic method may be very useful for detecting malignancies and predicting short-term outcomes, although larger-scale studies are needed.[19]
Use in bioengineering
RGD-based peptides have found many applications in biological research and medical devices. Culture plates coated with peptides mimicking ECM proteins' adhesion motifs, which promote prolonged culture of human embryonic stem cells, are on the market.[25] RGD is also a universally used tool in the construction of multifunctional "smart" materials, such as tumor-targeted nanoparticles.[26] Further, RGD is widely used in tissue engineering to promote tissue regeneration.[3]
Drug delivery
Conventional drug delivery methods, such as systemic or topical delivery, are associated with many issues such as low solubility, off-target effects, and disadvantageous pharmacokinetics. Nanoparticles have been employed to increase solubility and target delivery of the drug to the desired tissue, increasing concentration of the drug at the site of action and decreasing drug concentration elsewhere, thereby increasing the efficacy of the drug and decreasing side effects.[27][3] RGD has been employed to target nanoparticles containing drugs to specific cell types, especially cancer cells expressing integrin αvβ3.[3]
Many research groups utilize RGD to target the chemotherapeutic doxorubicin to cancer cells. Like other chemotherapeutics of its class, doxorubicin causes hair loss, nausea, vomiting, and myelosuppression, and can lead to cardiomyopathy and congestive heart failure. Clinically available Doxil utilizes liposomes to reduce accumulation of doxorubicin in myocardial tissue, thereby reducing cardiotoxicity.[28] However, such nanoparticles rely on passive targeting of tumors by the EPR effect, which varies by patient and tumor type.[28][29] Active targeting strategies aim to increase drug transport into cells to improve efficacy and counter multidrug resistance.[28]
In addition to doxorubicin, RGD-conjugated nanomaterials have been used to deliver the chemotherapeutics cisplatin, docetaxel, paclitaxel, 5-fluorouracil, and Gemcitabine to cancer cells. Such nanomaterials have also been used to deliver combination cytotoxic and vascular disrupting therapies.[3]
Gene delivery
While gene therapy has gained significant attention from the medical community, especially for cancer therapy, a lack of safe and efficient gene delivery vectors has become a bottleneck to clinical translation.[30] While viral vectors demonstrate high transfection efficiency and protect delivered genes, there are safety concerns associated with immune responses to the virus. Many nonviral vectors have been proposed, especially cationic lipids and polymers. However, these demonstrate low transfection efficiency compared to viruses. Therefore RGD has been coupled to nonviral vectors to target delivery of genetic material to the desired cells, thereby increasing transfection efficiency.[30]
Tissue engineering
Tissue engineering aims to replace lost or damaged tissues within the body. The success of such efforts has depended greatly upon the ability to direct cell behavior and encourage regeneration of tissues. A key method of doing so utilizes ECM-derived ligands such as RGD to control cellular responses to a biomaterial, such as attachment, proliferation, and differentiation.[31]
Vascular tissue
High rates of cardiovascular disease creates a high demand for grafts for vascular bypass surgery, especially small-diameter grafts which prevent occlusion.[3] Modifying vascular tissue grafts with RGD has been shown to inhibit platelet adhesion, improve cell infiltration and enhance endothelialization.[3] There have also been efforts to regenerate damaged heart tissues by applying cardiac patches following myocardial infarction.[32] The addition of RGD onto a cardiac tissue scaffold has been shown to promote cell adhesion, prevent apoptosis and enhance tissue regeneration.[33] RGD peptide has also been used to improve endothelial cell adhesion and proliferation on synthetic heart valves.[34]
Bone tissue
Bone defects or fractures can occur in a number of ways, including trauma, neoplasm, osteoporosis, or congenital disorders. Treatments such as autografts or allografts suffer from lack of donor sites and chance of communicable disease, respectively. There is therefore considerable interest in developing tissue engineered bone constructs, which should encourage tissue regeneration.[35] Coating an implant with RGD has been shown to improve bone cell adhesion, proliferation and survival. In vivo studies of such coatings additionally demonstrated improved osseointegration. Modifying a titanium implant surface with a protein containing RGD improved bone mineralization and implant integration and prevented failure of the prosthetic.[34]
Eye tissue
Damage to the cornea causes significant vision impairment, the most common treatment for which is allograft cornea transplantation. However, donor corneal grafts are in short supply and, like other tissue grafts, carry the risk of rejection or communicable disease.[36] Thus, tissue engineered options are desirable. In silk biomaterial scaffolds which replicate the hierarchical structure of the cornea, the addition of RGD improved cell attachment, alignment, proliferation, and ECM protein expression.[3][36] Additionally, RGD has been used in regeneration of retinal pigmented epithelium. This tissue can be generated from human embryonic and induced pluripotent stem cells, however with inefficient differentiation. It has been shown that RGD-alginate hydrogels improve derivation of retinal tissue from stem cells.[3][37]
Ligand presentation
RGD and other bioactive ligands can be presented on the surface of a biomaterial in a number of different spatial arrangements, and it has been demonstrated that these arrangements have a significant impact on cell behavior. In self-assembled monolayers, it was found that adhesion and proliferation of both human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (MSCs) increased as a function of RGD peptide density. These studies also showed that RGD density could change integrin expression, which has been postulated to enable control of biochemical signaling pathways. Further investigation of MSCs on self-assembled monolayers showed that modulating RGD density and the affinity of RGD for αvβ3 (through use of linear and cyclized RGD) could be used to control the differentiation of MSCs.[38] The effect of RGD presentation on cells in 3D biomaterials, which more accurately replicate the in vivo environment, has also been evaluated. In degradable polyethylene glycol hydrogels, the length of capillary-like structures formed by HUVECs was directly proportional to the density of RGD in the hydrogel.[38][39] Additionally, studies in nano-patterning have shown that, whereas an increase in global RGD density increases cell adhesion strength until saturation, an increase in local (mico/nano-scale) RGD density does not follow this trend.[38]
Alternatives
RGD is the most widely used of a larger class of cell adhesive peptides. These short amino acid sequences are the minimum motif of a larger protein that is necessary for binding to a cell surface receptor that drives cell adhesion.[40] The majority (89%) of published studies on biomaterials functionalized with cell adhesive peptides use RGD, whereas IKVAV and YIGSR are used in 6%, and 4% of those studies, respectively.[40] Cell adhesive peptides isolated from fibronectin include RGD, RGDS, PHSRN, and REDV.[41][42] YIGSR and IKVAV are isolated from laminin, whereas DGEA and GFOGER/GFPGER are isolated from collagen.[41] Artificial amino acid sequences, which bear no biological similarity to ECM proteins, have also been synthesized, and include the α5β1-specific peptide RRETAWA.[31][43]
| Peptide | Source | Receptor Integrin | Major uses | References |
|---|---|---|---|---|
| RGD(S) | Fibronectin | α3β1, α5β1, α8β1, αvβ1, αvβ3, αvβ5, αvβ6, αIIbβ3 | Promotes cell adhesion, targets tumors, used for drug discovery | ,[1][2][3][41] |
| PHSRN | Fibronectin | α5β1 | Synergistic for cell adhesion when covalently attached to RGD | ,[31][41] |
| REDV | Fibronectin | α4β1 | Promotes endothelial cell adhesion | ,[40][41] |
| YIGSR | Laminin | α4β1 | Promotes cell adhesion, inhibits angiogenesis and tumor growth | ,[40][41] |
| IKVAV | Laminin | α3β1 | Promotes cell adhesion and neurite outgrowth | ,[40][41] |
| DGEA | Collagen type I | α2β1 | Inhibits adhesion of platelets or adenocarcinoma to collagen | ,[40][41] |
| GFOGER/GFPGER | Collagen type I | α1β1 and α2β1 | Promotes osteogenesis in biomaterials | ,[1][40][41] |
| Eptifibatide | Derived from snake venom | αIIbβ3 | Thrombosis inhibition | [16] |
| RRETAWA | Synthetic | α5β1 | Promotes endothelial cell adhesion without platelet adhesion | ,[31][43] |
Chemical modifications
Linear RGD peptides suffer from low binding affinity, rapid degradation by proteases, and lack of specificity for integrin type.[44] RGD can be cyclized, or made into a cyclic compound, via disulfide, thioether, or rigid aromatic ring linkers. This leads to an increase in binding affinity and selectivity for integrin αVβ3 relative to αIIBβ3.[44][30] For example, the cyclic peptide ACDCRGDCFCG, also known as RGD4C, was shown to be 200-fold more potent than commonly used linear RGD peptides.[30] The structural rigidity of cyclic RGD peptides improves their binding properties and prevents degradation at the highly susceptible aspartic acid residue, thereby increasing their stability.[30] Many RGD derivative drugs and diagnostics are cyclized, including Eptifibatide, Cilengitide, CEND-1, and 18F-Galacto-RGD, and 18F-Fluciclatide-RGD.[13][18]
References
- ↑ 1.0 1.1 1.2 Plow, Edward F.; Haas, Thomas A.; Zhang, Li; Loftus, Joseph; Smith, Jeffrey W. (2000-07-21). "Ligand Binding to Integrins" (in en). Journal of Biological Chemistry 275 (29): 21785–21788. doi:10.1074/jbc.R000003200. ISSN 0021-9258. PMID 10801897.
- ↑ 2.0 2.1 2.2 Ley, Klaus; Rivera-Nieves, Jesus; Sandborn, William J.; Shattil, Sanford (2016). "Integrin-based therapeutics: biological basis, clinical use and new drugs". Nature Reviews Drug Discovery 15 (3): 173–183. doi:10.1038/nrd.2015.10. PMID 26822833.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Alipour, Mohsen; Baneshi, Marzieh; Hosseinkhani, Saman; Mahmoudi, Reza; Jabari Arabzadeh, Ali; Akrami, Mohammad; Mehrzad, Jalil; Bardania, Hassan (April 2020). "Recent progress in biomedical applications of RGD-based ligand: From precise cancer theranostics to biomaterial engineering: A systematic review". Journal of Biomedical Materials Research. Part A 108 (4): 839–850. doi:10.1002/jbm.a.36862. ISSN 1552-4965. PMID 31854488. https://pubmed.ncbi.nlm.nih.gov/31854488/.
- ↑ Pierschbacher, Michael D.; Ruoslahti, Erkki (1984-05-03). "Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule" (in en). Nature 309 (5963): 30–33. doi:10.1038/309030a0. PMID 6325925. Bibcode: 1984Natur.309...30P.
- ↑ Pytela, Robert; Pierschbacher, Michael D.; Ruoslahti, Erkki (1985). "Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor". Cell 40 (1): 191–198. doi:10.1016/0092-8674(85)90322-8. PMID 3155652.
- ↑ Pytela, R.; Pierschbacher, M. D.; Ginsberg, M. H.; Plow, E. F.; Ruoslahti, E. (1986-03-28). "Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors" (in en). Science 231 (4745): 1559–1562. doi:10.1126/science.2420006. ISSN 0036-8075. PMID 2420006. Bibcode: 1986Sci...231.1559P.
- ↑ Hynes, R (1987). "Integrins: A family of cell surface receptors". Cell 48 (4): 549–554. doi:10.1016/0092-8674(87)90233-9. PMID 3028640.
- ↑ Ruoslahti, E.; Pierschbacher, M. D. (1987-10-23). "New perspectives in cell adhesion: RGD and integrins" (in en). Science 238 (4826): 491–497. doi:10.1126/science.2821619. ISSN 0036-8075. PMID 2821619. Bibcode: 1987Sci...238..491R.
- ↑ Pierschbacher, M. D.; Ruoslahti, E. (1987-12-25). "Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion." (in en). Journal of Biological Chemistry 262 (36): 17294–17298. doi:10.1016/S0021-9258(18)45376-8. ISSN 0021-9258. PMID 3693352. http://www.jbc.org/content/262/36/17294.
- ↑ Humphries, M. J. (1990-12-01). "The molecular basis and specificity of integrin-ligand interactions" (in en). Journal of Cell Science 97 (4): 585–592. doi:10.1242/jcs.97.4.585. ISSN 0021-9533. PMID 2077034. http://jcs.biologists.org/content/97/4/585.
- ↑ Mas-Moruno, Carlos; Rechenmacher, Florian; Kessler, Horst (2010). "Cilengitide: The First Anti-Angiogenic Small Molecule Drug Candidate. Design, Synthesis and Clinical Evaluation". Anti-Cancer Agents in Medicinal Chemistry 10 (10): 753–768. doi:10.2174/187152010794728639. PMID 21269250.
- ↑ 12.0 12.1 Battle, Mark R.; Goggi, Julian L.; Allen, Lucy; Barnett, Jon; Morrison, Matthew S. (2011-03-01). "Monitoring Tumor Response to Antiangiogenic Sunitinib Therapy with 18F-Fluciclatide, an 18F-Labeled αVβ3-Integrin and αVβ5-Integrin Imaging Agent" (in en). Journal of Nuclear Medicine 52 (3): 424–430. doi:10.2967/jnumed.110.077479. ISSN 0161-5505. PMID 21321268.
- ↑ 13.0 13.1 Zeymer, Uwe; Wienbergen, Harm (2007-12-01). "A Review of Clinical Trials with Eptifibatide in Cardiology" (in en). Cardiovascular Drug Reviews 25 (4): 301–315. doi:10.1111/j.1527-3466.2007.00022.x. ISSN 1527-3466. PMID 18078431.
- ↑ Kloner, Robert A. (2013-08-02). "Current State of Clinical Translation of Cardioprotective Agents for Acute Myocardial Infarction" (in en). Circulation Research 113 (4): 451–463. doi:10.1161/circresaha.112.300627. ISSN 0009-7330. PMID 23908332.
- ↑ King, Shawn; Short, Marintha; Harmon, Cassidy (2016-03-01). "Glycoprotein IIb/IIIa inhibitors: The resurgence of tirofiban" (in en). Vascular Pharmacology 78: 10–16. doi:10.1016/j.vph.2015.07.008. ISSN 1537-1891. PMID 26187354. https://www.sciencedirect.com/science/article/pii/S1537189115001573.
- ↑ 16.0 16.1 Phillips, David R; Scarborough, Robert M (1997-08-18). "Clinical Pharmacology of Eptifibatide" (in en). The American Journal of Cardiology 80 (4, Supplement 1): 11B–20B. doi:10.1016/S0002-9149(97)00572-9. ISSN 0002-9149. PMID 9291241. https://www.sciencedirect.com/science/article/pii/S0002914997005729.
- ↑ Hashemzadeh, Mehrnoosh; Furukawa, Matthew; Goldsberry, Sarah; Movahed, Mohammad Reza (2008). "Chemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: A review". Experimental & Clinical Cardiology 13 (4): 192–197. ISSN 1205-6626. PMID 19343166.
- ↑ 18.0 18.1 Mas-Moruno, Carlos; Rechenmacher, Florian; Kessler, Horst (2010-12-01). "Cilengitide: The First Anti-Angiogenic Small Molecule Drug Candidate. Design, Synthesis and Clinical Evaluation". Anti-Cancer Agents in Medicinal Chemistry 10 (10): 753–768. doi:10.2174/187152010794728639. PMID 21269250. PMC 3267166. https://www.ingentaconnect.com/content/ben/acamc/2010/00000010/00000010/art00007.
- ↑ 19.0 19.1 19.2 Liu, Jie; Yuan, Shuanghu; Wang, Linlin; Sun, Xindong; Hu, Xudong; Meng, Xue; Yu, Jinming (2019-01-10). "Diagnostic and Predictive Value of Using RGD PET/CT in Patients with Cancer: A Systematic Review and Meta-Analysis" (in en). BioMed Research International 2019: e8534761. doi:10.1155/2019/8534761. ISSN 2314-6133. PMID 30733968.
- ↑ Al-Abd, Ahmed M.; Alamoudi, Abdulmohsin J.; Abdel-Naim, Ashraf B.; Neamatallah, Thikryat A.; Ashour, Osama M. (2017-11-01). "Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies – A review" (in en). Journal of Advanced Research 8 (6): 591–605. doi:10.1016/j.jare.2017.06.006. ISSN 2090-1232. PMID 28808589.
- ↑ Khasraw, Mustafa; Ameratunga, Malaka S; Grant, Robin; Wheeler, Helen; Pavlakis, Nick (2014-09-22). "Antiangiogenic therapy for high-grade glioma" (in en). Cochrane Database of Systematic Reviews (9): CD008218. doi:10.1002/14651858.cd008218.pub3. PMID 25242542.
- ↑ Dean, A.; Gill, S.; McGregor, M.; Broadbridge, V.; Jarvelainen, H. A.; Price, T. J. (2020-09-01). "1528P Phase I trial of the first-in-class agent CEND-1 in combination with gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer" (in English). Annals of Oncology 31: S941. doi:10.1016/j.annonc.2020.08.2011. ISSN 0923-7534. https://www.annalsofoncology.org/article/S0923-7534(20)42007-1/abstract.
- ↑ "A Phase 1 Clinical Trial of CEND-1 in Combination with Nabpaclitaxel and Gemcitabine in Metastatic Exocrine Pancreatic Cancer". 24 October 2020. https://clinicaltrials.gov/ct2/show/NCT03517176.
- ↑ 24.0 24.1 Chen, Haojun; Niu, Gang; Wu, Hua; Chen, Xiaoyuan (2016-01-01). "Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3". Theranostics 6 (1): 78–92. doi:10.7150/thno.13242. ISSN 1838-7640. PMID 26722375.
- ↑ Villa-Diaz, L. G.; Ross, A. M.; Lahann, J.; Krebsbach, P. H. (2013). "Concise Review: The Evolution of human pluripotent stem cell culture: From feeder cells to synthetic coatings" (in en). Stem Cells 31 (1): 1–7. doi:10.1002/stem.1260. ISSN 1549-4918. PMID 23081828.
- ↑ Ruoslahti, Erkki (2012-07-24). "Peptides as Targeting Elements and Tissue Penetration Devices for Nanoparticles" (in en). Advanced Materials 24 (28): 3747–3756. doi:10.1002/adma.201200454. ISSN 1521-4095. PMID 22550056.
- ↑ Abasian, Payam; Ghanavati, Sonya; Rahebi, Saeed; Khorasani, Saied Nouri; Khalili, Shahla (2020). "Polymeric nanocarriers in targeted drug delivery systems: A review" (in en). Polymers for Advanced Technologies 31 (12): 2939–2954. doi:10.1002/pat.5031. ISSN 1099-1581. https://onlinelibrary.wiley.com/doi/abs/10.1002/pat.5031.
- ↑ 28.0 28.1 28.2 Sun, Yuan; Kang, Chen; Liu, Fei; Zhou, You; Luo, Lei; Qiao, Hongzhi (2017). "RGD Peptide-Based Target Drug Delivery of Doxorubicin Nanomedicine" (in en). Drug Development Research 78 (6): 283–291. doi:10.1002/ddr.21399. ISSN 1098-2299. PMID 28815721. https://onlinelibrary.wiley.com/doi/abs/10.1002/ddr.21399.
- ↑ Shi, Yang; van der Meel, Roy; Chen, Xiaoyuan; Lammers, Twan (2020). "The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy" (in en). Theranostics 10 (17): 7921–7924. doi:10.7150/thno.49577. ISSN 1838-7640. PMID 32685029. PMC 7359085. https://www.thno.org/v10p7921.htm.
- ↑ 30.0 30.1 30.2 30.3 30.4 Park, J.; Singha, K.; Son, S.; Kim, J.; Namgung, R.; Yun, C.-O.; Kim, W. J. (November 2012). "A review of RGD-functionalized nonviral gene delivery vectors for cancer therapy" (in en). Cancer Gene Therapy 19 (11): 741–748. doi:10.1038/cgt.2012.64. ISSN 1476-5500. PMID 23018622.
- ↑ 31.0 31.1 31.2 31.3 Dhavalikar, Prachi; Robinson, Andrew; Lan, Ziyang; Jenkins, Dana; Chwatko, Malgorzata; Salhadar, Karim; Jose, Anupriya; Kar, Ronit et al. (2020). "Review of Integrin-Targeting Biomaterials in Tissue Engineering" (in en). Advanced Healthcare Materials 9 (23): 2000795. doi:10.1002/adhm.202000795. ISSN 2192-2659. PMID 32940020.
- ↑ Pomeroy, Jordan E.; Helfer, Abbigail; Bursac, Nenad (2020-09-01). "Biomaterializing the promise of cardiac tissue engineering" (in en). Biotechnology Advances 42: 107353. doi:10.1016/j.biotechadv.2019.02.009. ISSN 0734-9750. PMID 30794878.
- ↑ Shachar, Michal; Tsur-Gang, Orna; Dvir, Tal; Leor, Jonathan; Cohen, Smadar (2011-01-01). "The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering" (in en). Acta Biomaterialia 7 (1): 152–162. doi:10.1016/j.actbio.2010.07.034. ISSN 1742-7061. PMID 20688198. https://www.sciencedirect.com/science/article/pii/S1742706110003545.
- ↑ 34.0 34.1 Jana, Soumen (2019-11-01). "Endothelialization of cardiovascular devices" (in en). Acta Biomaterialia 99: 53–71. doi:10.1016/j.actbio.2019.08.042. ISSN 1742-7061. PMID 31454565. https://www.sciencedirect.com/science/article/pii/S1742706119305987.
- ↑ Venkatesan, Jayachandran; Bhatnagar, Ira; Manivasagan, Panchanathan; Kang, Kyong-Hwa; Kim, Se-Kwon (2015-01-01). "Alginate composites for bone tissue engineering: A review" (in en). International Journal of Biological Macromolecules 72: 269–281. doi:10.1016/j.ijbiomac.2014.07.008. ISSN 0141-8130. PMID 25020082. https://www.sciencedirect.com/science/article/pii/S0141813014004735.
- ↑ 36.0 36.1 Gil, Eun Seok; Mandal, Biman B.; Park, Sang-Hyug; Marchant, Jeffrey K.; Omenetto, Fiorenzo G.; Kaplan, David L. (2010-12-01). "Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering" (in en). Biomaterials 31 (34): 8953–8963. doi:10.1016/j.biomaterials.2010.08.017. ISSN 0142-9612. PMID 20801503.
- ↑ Hunt, Nicola C.; Hallam, Dean; Karimi, Ayesha; Mellough, Carla B.; Chen, Jinju; Steel, David H. W.; Lako, Majlinda (2017-02-01). "3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development" (in en). Acta Biomaterialia 49: 329–343. doi:10.1016/j.actbio.2016.11.016. ISSN 1742-7061. PMID 27826002.
- ↑ 38.0 38.1 38.2 Satav, Tushar; Huskens, Jurriaan; Jonkheijm, Pascal (2015). "Effects of Variations in Ligand Density on Cell Signaling" (in en). Small 11 (39): 5184–5199. doi:10.1002/smll.201500747. ISSN 1613-6829. PMID 26292200. https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.201500747.
- ↑ Nguyen, Eric H.; Zanotelli, Matthew R.; Schwartz, Michael P.; Murphy, William L. (2014-02-01). "Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays" (in en). Biomaterials 35 (7): 2149–2161. doi:10.1016/j.biomaterials.2013.11.054. ISSN 0142-9612. PMID 24332391.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 Huettner, Nick; Dargaville, Tim R.; Forget, Aurelien (2018-04-01). "Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD" (in en). Trends in Biotechnology 36 (4): 372–383. doi:10.1016/j.tibtech.2018.01.008. ISSN 0167-7799. PMID 29422411. https://www.sciencedirect.com/science/article/pii/S0167779918300295.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 41.8 Hamley, I. W. (2017-12-11). "Small Bioactive Peptides for Biomaterials Design and Therapeutics". Chemical Reviews 117 (24): 14015–14041. doi:10.1021/acs.chemrev.7b00522. ISSN 0009-2665. PMID 29227635. https://pubs.acs.org/doi/pdf/10.1021/acs.chemrev.7b00522.
- ↑ Kakinoki, Sachiro; Yamaoka, Tetsuji (2015-04-15). "Single-Step Immobilization of Cell Adhesive Peptides on a Variety of Biomaterial Substrates via Tyrosine Oxidation with Copper Catalyst and Hydrogen Peroxide". Bioconjugate Chemistry 26 (4): 639–644. doi:10.1021/acs.bioconjchem.5b00032. ISSN 1043-1802. PMID 25742028. https://doi.org/10.1021/acs.bioconjchem.5b00032.
- ↑ 43.0 43.1 Meyers, Steven R.; Grinstaff, Mark W. (2011-10-18). "Biocompatible and Bioactive Surface Modifications for Prolonged In Vivo Efficacy". Chemical Reviews 112 (3): 1615–1632. doi:10.1021/cr2000916. ISSN 0009-2665. PMID 22007787.
- ↑ 44.0 44.1 Shi, Jiyun; Wang, Fan; Liu, Shuang (2016-02-01). "Radiolabeled cyclic RGD peptides as radiotracers for tumor imaging" (in en). Biophysics Reports 2 (1): 1–20. doi:10.1007/s41048-016-0021-8. ISSN 2364-3420. PMID 27819026. PMC 5071373. https://doi.org/10.1007/s41048-016-0021-8.
 |