Chemistry:Alginic acid
| |
| Names | |
|---|---|
| Other names
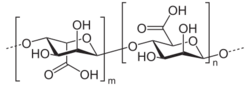
Alginic acid; E400; [D-ManA(β1→4)L-GulA(α1→4)]n
| |
| Identifiers | |
| ChemSpider |
|
| EC Number |
|
| UNII | |
| Properties | |
| (C6H8O6)n | |
| Molar mass | 10,000 – 600,000 |
| Appearance | White to yellow, fibrous powder |
| Density | 1.601 g/cm3 |
| Acidity (pKa) | 1.5–3.5 |
| Pharmacology | |
| 1=ATC code }} | A02BX13 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

Alginic acid, also called algin, is a naturally occurring, edible polysaccharide found in brown algae. It is hydrophilic and forms a viscous gum when hydrated. When the alginic acid binds with sodium and calcium ions, the resulting salts are known as alginates. Its colour ranges from white to yellowish-brown. It is sold in filamentous, granular, or powdered forms.
It is a significant component of the biofilms produced by the bacterium Pseudomonas aeruginosa, a major pathogen found in the lungs of some people who have cystic fibrosis.[1] The biofilm and P. aeruginosa have a high resistance to antibiotics,[2] but are susceptible to inhibition by macrophages.[3]
Alginate was discovered by British chemical scientist E. C. C. Stanford in 1881, and he patented an extraction process for it in the same year.[4] The alginate was extracted, in the original patent, by first soaking the algae in water or diluted acid, then extracting the alginate by soaking it in sodium carbonate, and finally precipitating the alginate from solution.[5][better source needed]
Structure
Forms
Alginates are refined from brown seaweeds. Throughout the world, many of the Phaeophyceae class brown seaweeds are harvested to be processed and converted into sodium alginate. Sodium alginate is used in many industries including food, animal food, fertilisers, textile printing, and pharmaceuticals. Dental impression material uses alginate as its means of gelling. Food grade alginate is an approved ingredient in processed and manufactured foods.[6]
Brown seaweeds range in size from the giant kelp Macrocystis pyrifera which can be 20–40 meters long, to thick, leather-like seaweeds from 2–4 m long, to smaller species 30–60 cm long. Most brown seaweed used for alginates are gathered from the wild, with the exception of Laminaria japonica, which is cultivated in China for food and its surplus material is diverted to the alginate industry in China.
Alginates from different species of brown seaweed vary in their chemical structure, resulting in different physical properties of alginates. Some species yield an alginate that gives a strong gel, another a weaker gel, some may produce a cream or white alginate, while others are difficult to gel and are best used for technical applications where color does not matter.[7]
Commercial grade alginate is extracted from giant kelp Macrocystis pyrifera, Ascophyllum nodosum, and types of Laminaria. Alginates are also produced by two bacterial genera Pseudomonas and Azotobacter, which played a major role in the unravelling of its biosynthesis pathway. Bacterial alginates are useful for the production of micro- or nanostructures suitable for medical applications.[8]
Sodium alginate (NaC6H7O6) is the sodium salt of alginic acid. Sodium alginate is a gum.
Potassium alginate (KC6H7O6) is the potassium salt of alginic acid.
Calcium alginate (CaC12H14O12) is the calcium salt of alginic acid. It is made by replacing the sodium ion in sodium alginate with a calcium ion (ion exchange).
Production
The manufacturing process used to extract sodium alginates from brown seaweed fall into two categories: 1) calcium alginate method and, 2) alginic acid method.[clarification needed]
Chemically the process is simple, but difficulties arise from the physical separations required between the slimy residues from viscous solutions and the separation of gelatinous precipitates that hold large amounts of liquid within their structure, so they resist filtration and centrifugation.[9] The conventional process involves large amounts of reagents and solvents, as well as time-consuming steps.[4] Simpler and newer techniques, such as microwave-assisted extraction, ultrasound, high pressure, pressurized fluid extraction, and enzyme-assisted extraction, are the subject of research.[4]
The most common, conventional extraction process involves six steps: pre-treatment of the algal biomass, acid treatment, alkaline extraction, precipitation, bleaching, and drying.[4] Pre-treatments mainly aim at either breaking the cell wall to help extract the alginate, or removing other compounds and contaminants from the algae.[4] Drying is of the first kind, also helping to prevent bacterial growth; algae which is dried is also usually powdered to expose more surface area.[4] Common treatments to remove contaminants include treatments with ethanol and formaldehyde, the latter of which is very common; ethanol solutions help remove compounds bonded to the alginate, and formaldehyde solutions help prevent enzymatic or microbial reactions.[4]
The algae is then treated with an acidic solution to help disrupt cell walls, which converts the alginate salts into insoluble alginic acid; a subsequently applied alkaline solution (pH 9-10), usually sodium carbonate, converts it back into water-soluble sodium alginate, which is then precipitated.[4] It is also possible to extract the alginate directly with an alkaline treatment, but this is less common.[4]
Alginic acid is usually precipitated, through different techniques, with either an alcohol (usually ethanol), calcium chloride, or hydrochloric acid.[4] After the alginin is precipitated into a fine paste, it is dried, ground to the desired grain size, and finally purified through a variety of techniques.[4] Commercial alginate for biomedical and pharmaceutical use is extracted and purified through more rigorous techniques, but these are trade secrets.[4]
Derivatives
Various alginate-based materials can be produced, including porous scaffold material, alginate hydrogel, nonwoven fabric, and alginate membranes.[10] Techniques used to produce these include ion cross-linking, microfluidic spinning, freeze drying, wet spinning, and immersive centrifugal jet spinning.[10]
Calcium salts added to a sodium alginate solution to induce ionic cross-linking, which produces the hydrogel. Freeze-drying the hydrogel to eliminate water produces the porous scaffold material.[10]
Wet spinning consists of extruding an alginate solution from a spinneret into a calcium salt solution to induce ionic cross-linking (forming the gel), and then drawing the fibers out of the bath with draft rollers. Microfluidic spinning, a simpler and more eco-friendly implementation of the process, involves introducing calcium salt flows flowing alongside and touching a central "core" flow of alginate. These flows form a "sheath". The fiber then emerges from the core flow. This technique can be used to produce shaped and grooved fibers.[10]

Alginate fiber, which is used in fabric, is usually produced through either microfluidic spinning or wet spinning, or electrospinning to obtain thinner fibers.[10] The fabric, which can be used in wound dressing and other applications, is produced by carding and then needle punching [clarification needed] the fibers.[10]
Uses
As of 2022, alginate had become one of the most preferred materials as an abundant natural biopolymer.[10] It is particularly useful as a biomaterial because of its nontoxicity, hygroscopicity, and biocompatibility, and can imitate local bioenvironments; its degradation product can be easily cleared by the kidneys.[10]
Alginate is also used for waterproofing and fireproofing fabrics, in the food industry as a thickening agent for drinks, ice cream, cosmetics, as a gelling agent for jellies, known by the code E401 and sausage casing.[11][12] Sodium alginate is mixed with soybean protein to make meat analogue.[13]
Sodium alginate is used in reactive dye printing and as a thickener for reactive dyes in textile screen-printing. Alginates do not react with these dyes and wash out easily, unlike starch-based thickeners. It also serves as a material for micro-encapsulation.[14]
Calcium alginate is used in different types of medical products, including skin wound dressings to promote healing,[15][16] and may be removed with less pain than conventional dressings.
Alginate hydrogels
In research on bone reconstruction, alginate composites have favorable properties encouraging regeneration, such as improved porosity, cell proliferation, and mechanical strength.[17] Alginate hydrogel is a common biomaterial for bio-fabrication of scaffolds and tissue regeneration.[18]
Covalent bonding of thiol groups to alginate improves in-situ gelling and mucoadhesive properties; the thiolated polymer (thiomer) forms disulfide bonds within its polymeric network and with cysteine-rich subdomains of the mucus layer.[19] Thiolated alginates are used as in situ gelling hydrogels,[20] and are under preliminary research as possible mucoadhesive drug delivery systems.[21] Alginate hydrogels may be used for drug delivery, exhibiting responses to pH changes, temperature changes, redox, and the presence of enzymes.[22]
See also
- Hyaluronic acid: a polysaccharide in animals.
- Agar
References
- ↑ Davies, JC (2002). "Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence.". Paediatric Respiratory Reviews 3 (2): 128–34. doi:10.1016/S1526-0550(02)00003-3. ISSN 1526-0542. PMID 12297059.
- ↑ Boyd, A; Chakrabarty, AM (1995). "Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide.". Journal of Industrial Microbiology 15 (3): 162–8. doi:10.1007/BF01569821. ISSN 0169-4146. PMID 8519473.
- ↑ Leid, JG; Willson, CJ; Shirtliff, ME; Hassett, DJ; Parsek, MR; Jeffers, AK (1 November 2005). "The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing.". Journal of Immunology 175 (11): 7512–8. doi:10.4049/jimmunol.175.11.7512. ISSN 0022-1767. PMID 16301659. http://www.jimmunol.org/content/175/11/7512.full.pdf.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Bojorges, Hylenne; López-Rubio, Amparo; Martínez-Abad, Antonio; Fabra, María José (2023-10-01). "Overview of alginate extraction processes: Impact on alginate molecular structure and techno-functional properties". Trends in Food Science & Technology 140. doi:10.1016/j.tifs.2023.104142. ISSN 0924-2244. https://www.sciencedirect.com/science/article/pii/S0924224423002571.
- ↑ Pereira, Leonel; Cotas, João (2020-02-05), "Introductory Chapter: Alginates - A General Overview" (in en), Alginates - Recent Uses of This Natural Polymer (IntechOpen), ISBN 978-1-78985-642-2, https://www.intechopen.com/chapters/68305, retrieved 2024-08-06
- ↑ "Alginates". Agricultural Marketing Service, US Department of Agriculture. 5 February 2015. https://www.ams.usda.gov/sites/default/files/media/Alginates%20TR%202015.pdf.
- ↑ FAO fisheries technical paper 441, Tevita Bainiloga Jnr, School of Chemistry, University College, University of New South Wales and Australian Defence Force Academy Canberra Australia
- ↑ Remminghorst and Rehm (2009). "Microbial Production of Alginate: Biosynthesis and Applications". Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press. ISBN 978-1-904455-36-3.
- ↑ FAO Fisheries Technical Paper, 2003
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 Zhang, Xiaolin; Wang, Xinran; Fan, Wei; Liu, Yi; Wang, Qi; Weng, Lin (2022-08-08). "Fabrication, Property and Application of Calcium Alginate Fiber: A Review". Polymers 14 (15): 3227. doi:10.3390/polym14153227. ISSN 2073-4360. PMID 35956740.
- ↑ "What is Sodium Alginate (E401) in food? Properties, Uses, Safety". FOODADDITIVES. 14 May 2020. https://foodadditives.net/thickeners/sodium-alginate/.
- ↑ Qin, Yimin (17 July 2018). Bioactive Seaweeds for Food Applications. doi:10.1016/C2016-0-04566-7. ISBN 978-0-12-813312-5. https://www.sciencedirect.com/topics/food-science/sausage-casing.
- ↑ Arasaki, Seibin; Arasaki, Teruko (January 1983). Low Calorie, High Nutrition Vegetables from the Sea (1st ed.). Tokyo, Japan: Japan Publications, Inc.. p. 35. ISBN 0-87040-475-X.
- ↑ Aizpurua-Olaizola, Oier; Navarro, Patricia; Vallejo, Asier; Olivares, Maitane; Etxebarria, Nestor; Usobiaga, Aresatz (2016-01-01). "Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes". Food Chemistry 190: 614–621. doi:10.1016/j.foodchem.2015.05.117. PMID 26213018. https://figshare.com/articles/journal_contribution/5028350.
- ↑ Lansdown AB (2002). "Calcium: a potential central regulator in wound healing in the skin". Wound Repair Regen 10 (5): 271–85. doi:10.1046/j.1524-475x.2002.10502.x. PMID 12406163.
- ↑ Stubbe, Birgit; Mignon, Arn; Declercq, Heidi; Vlierberghe, Sandra Van; Dubruel, Peter (2019). "Development of Gelatin-Alginate Hydrogels for Burn Wound Treatment" (in en). Macromolecular Bioscience 19 (8). doi:10.1002/mabi.201900123. ISSN 1616-5195. PMID 31237746. https://lirias.kuleuven.be/bitstream/123456789/663375/3/Development%20of%20Gelatin-Alginate%20Hydrogels%20for%20Burn%20Wound%20Treatment.docx.
- ↑ Venkatesan, J; Bhatnagar, I; Manivasagan, P; Kang, K. H.; Kim, S. K. (2015). "Alginate composites for bone tissue engineering: A review". International Journal of Biological Macromolecules 72: 269–81. doi:10.1016/j.ijbiomac.2014.07.008. PMID 25020082.
- ↑ Rastogi, Prasansha; Kandasubramanian, Balasubramanian (2019-09-10). "Review of alginate-based hydrogel bioprinting for application in tissue engineering" (in en). Biofabrication 11 (4): 042001. doi:10.1088/1758-5090/ab331e. ISSN 1758-5090. PMID 31315105. Bibcode: 2019BioFa..11d2001R.
- ↑ Leichner, C; Jelkmann, M; Bernkop-Schnürch, A (2019). "Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature". Adv Drug Deliv Rev 151-152: 191–221. doi:10.1016/j.addr.2019.04.007. PMID 31028759.
- ↑ Xu, G; Cheng, L; Zhang, Q; Sun, Y; Chen, C; Xu, H; Chai, Y; Lang, M (2016). "In situ thiolated alginate hydrogel: Instant formation and its application in hemostasis". J Biomater Appl 31 (5): 721–729. doi:10.1177/0885328216661557. PMID 27485953.
- ↑ Kassem, AA; Issa, DA; Kotry, GS; Farid, RM (2017). "Thiolated alginate-based multiple layer mucoadhesive films of metformin for intra-pocket local delivery: in vitro characterization and clinical assessment". Drug Dev. Ind. Pharm. 43 (1): 120–131. doi:10.1080/03639045.2016.1224895. PMID 27589817.
- ↑ Abasalizadeh, Farhad; Moghaddam, Sevil; Alizadeh, Effat; Fazljou, Mohammad; Torbati, Mohammadali; Akbarzadeh, Abolfazl (13 March 2020). "Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting". Journal of Biological Engineering 14 (8): 8. doi:10.1186/s13036-020-0227-7. PMID 32190110.
External links
 |
