Chemistry:Bismuth subgallate
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
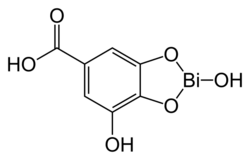
| Formula | C7H5BiO6 |
| Molar mass | 394.091 g·mol−1 |
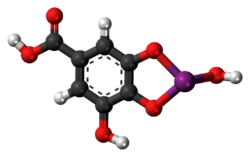
| 3D model (JSmol) | |
| Density | 1.1 g/cm3 |
| |
| |
| | |
Bismuth subgallate, with a chemical formula C7H5BiO6, is commonly used to treat malodor by deodorizing flatulence and stools. In the United States, it (bismuth subgallate) is the active ingredient in Devrom (internal deodorant), an over-the-counter FDA-approved medicine. Also, it has been used to treat Helicobacter pylori infection and is used in wound therapy. As an internal deodorant, it is commonly used by individuals who have had gastrointestinal stoma surgery, bariatric surgery, fecal incontinence, and irritable bowel syndrome.[1]
Also, a double blind study in 1974 reported its effectiveness as a flatulence/stool deodorant in ileostomy patients.[2]
Adverse effects
It can cause darkening of the tongue and stools, which is temporary.[3]
In 1974, a reversible encephalopathy was noted and examined in four colon cancer patients taking bismuth subgallate after abdominoperineal resection.[4]
Bismuth subgallate is contraindicated in case of hypersensitivity to the substance, and should be used with caution in people with liver disease or kidney disease.[3] It is grouped in pregnancy category C[3] (risk not ruled out: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks). During lactation, very little bismuth subgallate passes over to the child.[3]
Structure
File:Structure of bismuth subgallate.tif
Crystal structure determination of bismuth subgallate revealed it is a coordination polymer with the formula [Bi(C6H2(O)3COOH)(H2O)]n2nH2O.[5] The phenolate oxygen atoms of the gallate ligand chelate to bismuth cations and form chains. The material is nanoporous and the open-channels can be filled with small gas molecules such as carbon dioxide.[5]
See also
External links
- American Cancer Society: Ileostomy Guide [1]
- Cleveland Clinic-Having an Ileostomy– A Primer for New Ostomates [2]
- The Ostomy Files:The Issue of Oral Medications and a Fecal Ostomy [3]
- Devrom website [4]
References
- ↑ "Bismuth therapy in gastrointestinal diseases". Gastroenterology 99 (3): 863–75. September 1990. doi:10.1016/0016-5085(90)90983-8. PMID 2199292.
- ↑ "Correspondence: Bismuth subgallate as an effective means for the control of ileostomy odor: a double blind study". Gastroenterology 66 (3): 476. March 1974. doi:10.1016/S0016-5085(74)80150-2. PMID 4813513.
- ↑ 3.0 3.1 3.2 3.3 "Bismuth subgallate (OTC) Devrom". http://reference.medscape.com/drug/bismuth-subgallate-999413.
- ↑ "Reversible encephalopathy possibly associated with bismuth subgallate ingestion". British Medical Journal 1 (5901): 220–3. February 1974. doi:10.1136/bmj.1.5901.220. PMID 4818163.
- ↑ 5.0 5.1 5.2 "Elucidation of the elusive structure and formula of the active pharmaceutical ingredient bismuth subgallate by continuous rotation electron diffraction". Chemical Communications 53 (52): 7018–7021. July 2017. doi:10.1039/C7CC03180G. PMID 28613325.
 |

