Chemistry:Bismuth(III) oxide

| |
| |
| Names | |
|---|---|
| IUPAC names | |
| Other names
Bismuth oxide, bismuth sesquioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Bi2O3 | |
| Molar mass | 465.958 g·mol−1 |
| Appearance | yellow crystals or powder |
| Odor | odorless |
| Density | 8.90 g/cm3, solid |
| Melting point | 817 °C (1,503 °F; 1,090 K)[1] |
| Boiling point | 1,890 °C (3,430 °F; 2,160 K) |
| insoluble | |
| Solubility | soluble in acids |
| -83.0·10−6 cm3/mol | |
| Structure | |
| monoclinic, mP20, Space group P21/c (No 14) | |
| pseudo-octahedral | |
| Hazards | |
| Safety data sheet | MallBaker MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335, H413 | |
| P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Bismuth trisulfide Bismuth selenide Bismuth telluride |
Other cations
|
Dinitrogen trioxide Phosphorus trioxide Arsenic trioxide Antimony trioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite (tetragonal, much more rare), but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.[1]
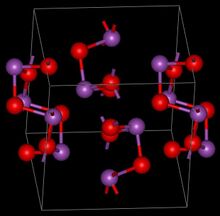
Structure
The structures adopted by Bi
2O
3 differ substantially from those of arsenic(III) oxide, As
2O
3, and antimony(III) oxide, Sb
2O
3.[2]
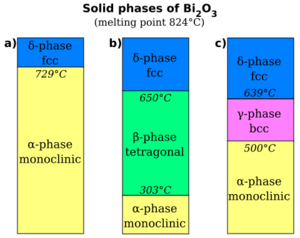
2O
3 as a function of temperature. (a) The α-phase transforms to the δ-phase when heated above 727 °C, which remains the structure until the melting point, 824 °C, is reached. When cooled, the δ-phase transforms into either the β-phase at 650 °C, shown in (b), or the γ-phase at 639 °C, shown in (c). The β-phase transforms to the α-phase at 303 °C. The γ-phase may persist to room temperature when the cooling rate is very slow, otherwise it transforms to the α-phase at 500 °C.[2]
Bismuth oxide, Bi
2O
3 has five crystallographic polymorphs. The room temperature phase, α-Bi
2O
3 has a monoclinic crystal structure. There are three high temperature phases, a tetragonal β-phase, a body-centred cubic γ-phase, a cubic δ-Bi
2O
3 phase and an ε-phase.
The room temperature α-phase has a complex structure with layers of oxygen atoms with layers of bismuth atoms between them. The bismuth atoms are in two different environments which can be described as distorted 6 and 5 coordinate respectively.[3]
β-Bi
2O
3 has a structure related to fluorite.[2]
γ-Bi
2O
3 has a structure related to that of sillenite (Bi
12SiO
20), but in which a small fraction of the bismuth atoms occupy positions occupied by silicon atoms in sillenite, so the formula may be written as Bi
12Bi
0.8O
19.2. The crystals are chiral (space group I23, or no. 197) with two Bi
12Bi
0.8O
19.2 formulas per unit cell.[4]
δ-Bi
2O
3 has a defective fluorite-type crystal structure in which two of the eight oxygen sites in the unit cell are vacant.[5]
ε-Bi
2O
3 has a structure related to the α- and β- phases but as the structure is fully ordered it is an ionic insulator. It can be prepared by hydrothermal means and transforms to the α- phase at 400 °C.[4]
The monoclinic α-phase transforms to the cubic δ-Bi
2O
3 when heated above 729 °C, which remains the structure until the melting point, 824 °C, is reached. The behaviour of Bi
2O
3 on cooling from the δ-phase is more complex, with the possible formation of two intermediate metastable phases; the tetragonal β-phase or the body-centred cubic γ-phase. The γ-phase can exist at room temperature with very slow cooling rates, but α-Bi
2O
3 always forms on cooling the β-phase. Even though when formed by heat, it reverts to α-Bi
2O
3 when the temperature drops back below 727 °C, δ-Bi
2O
3 can be formed directly through electrodeposition and remain relatively stable at room temperature, in an electrolyte of bismuth compounds that is also rich in sodium or potassium hydroxide so as to have a pH near 14.
Conductivity
The α-phase exhibits p-type electronic conductivity (the charge is carried by positive holes) at room temperature which transforms to n-type conductivity (charge is carried by electrons) between 550 °C and 650 °C, depending on the oxygen partial pressure.
The conductivity in the β, γ and δ-phases is predominantly ionic with oxide ions being the main charge carrier. Of these δ-Bi
2O
3 has the highest reported conductivity. At 750 °C the conductivity of δ-Bi
2O
3 is typically about 1 S cm−1, about three orders of magnitude greater than the intermediate phases and four orders greater than the monoclinic phase.
δ-Bi
2O
3 has a defective fluorite-type crystal structure in which two of the eight oxygen sites in the unit cell are vacant. These intrinsic vacancies are highly mobile due to the high polarisability of the cation sub-lattice with the 6s2 lone pair electrons of Bi3+. The Bi–O bonds have covalent bond character and are therefore weaker than purely ionic bonds, so the oxygen ions can jump into vacancies more freely.
The arrangement of oxygen atoms within the unit cell of δ-Bi
2O
3 has been the subject of much debate in the past. Three different models have been proposed. Sillén (1937) used powder X-ray diffraction on quenched samples and reported the structure of Bi
2O
3 was a simple cubic phase with oxygen vacancies ordered along <111>, the cube body diagonal.[6] Gattow and Schroder (1962) rejected this model, preferring to describe each oxygen site (8c site) in the unit cell as having 75% occupancy. In other words, the six oxygen atoms are randomly distributed over the eight possible oxygen sites in the unit cell. Currently, most experts seem to favour the latter description as a completely disordered oxygen sub-lattice accounts for the high conductivity in a better way.[7]
Willis (1965) used neutron diffraction to study the fluorite (CaF
2) system. He determined that it could not be described by the ideal fluorite crystal structure, rather, the fluorine atoms were displaced from regular 8c positions towards the centres of the interstitial positions.[8] Shuk et al. (1996)[9] and Sammes et al. (1999)[10] suggest that because of the high degree of disorder in δ-Bi
2O
3, the Willis model could also be used to describe its structure.
Use in solid-oxide fuel cells (SOFCs)
Interest has centred on δ-Bi
2O
3 as it is principally an ionic conductor. In addition to electrical properties, thermal expansion properties are very important when considering possible applications for solid electrolytes. High thermal expansion coefficients represent large dimensional variations under heating and cooling, which would limit the performance of an electrolyte. The transition from the high-temperature δ-Bi
2O
3 to the intermediate β-Bi
2O
3 is accompanied by a large volume change and consequently, a deterioration of the mechanical properties of the material. This, combined with the very narrow stability range of the δ-phase (727–824 °C), has led to studies on its stabilization to room temperature.
Bi
2O
3 easily forms solid solutions with many other metal oxides. These doped systems exhibit a complex array of structures and properties dependent on the type of dopant, the dopant concentration and the thermal history of the sample. The most widely studied systems are those involving rare earth metal oxides, Ln
2O
3, including yttria, Y
2O
3. Rare earth metal cations are generally very stable, have similar chemical properties to one another and are similar in size to Bi3+, which has a radius of 1.03 Å, making them all excellent dopants. Furthermore, their ionic radii decrease fairly uniformly from La3+ (1.032 Å), through Nd3+ (0.983 Å), Gd3+ (0.938 Å), Dy3+ (0.912 Å) and Er3+ (0.89 Å), to Lu3+ (0.861 Å) (known as the "lanthanide contraction"), making them useful to study the effect of dopant size on the stability of the Bi
2O
3 phases.
Bi
2O
3 has also been used as sintering additive in the Sc
2O
3-doped zirconia system for intermediate temperature SOFC.[11]
Preparation
The trioxide can be prepared by ignition of bismuth hydroxide.[1] Bismuth trioxide can be also obtained by heating bismuth subcarbonate at approximately 400 °C.[12]
Reactions
Atmospheric carbon dioxide or CO
2 dissolved in water readily reacts with Bi
2O
3 to generate bismuth subcarbonate.[12] Bismuth oxide is considered a basic oxide, which explains the high reactivity with CO
2. However, when acidic cations such as Si(IV) are introduced within the structure of the bismuth oxide, the reaction with CO
2 do not occur.[12]
Bismuth(III) oxide reacts with a mixture of concentrated aqueous sodium hydroxide and bromine or aqueous potassium hydroxide and bromine to form sodium bismuthate or potassium bismuthate, respectively.[13]
Usage
Medical devices
Bismuth oxide is occasionally used in dental materials to make them more opaque to X-rays than the surrounding tooth structure. In particular, bismuth (III) oxide has been used in hydraulic silicate cements (HSC), originally in "MTA" (a trade name, standing for the chemically-meaningless "mineral trioxide aggregate") from 10 to 20% by mass with a mixture of mainly di- and tri-calcium silicate powders. Such HSC is used for dental treatments such as: apicoectomy, apexification, pulp capping, pulpotomy, pulp regeneration, internal repair of iatrogenic perforations, repair of resorption perforations, root canal sealing and obturation. MTA sets into a hard filling material when mixed with water. Some resin-based materials also include an HSC with bismuth oxide. Problems have allegedly arisen with bismuth oxide because it is claimed not to be inert at high pH, specifically that it slows the setting of the HSC, but also over time can lose color[14] by exposure to light or reaction with other materials that may have been used in the tooth treatment, such as sodium hypochlorite.[15]
Radiative cooling
Bismuth oxide was used to develop a scalable colored surface high in solar reflectance and heat emissivity for passive radiative cooling. The paint was non-toxic and demonstrated a reflectance of 99% and emittance of 97%. In field tests the coating exhibited significant cooling power and reflected potential for the further development of colored surfaces practical for large-scale radiative cooling applications.[16]
References
- ↑ 1.0 1.1 1.2 Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. p. 243. ISBN 0-07-049439-8. https://books.google.com/books?id=Xqj-TTzkvTEC&pg=PA243. Retrieved 2009-06-06.
- ↑ 2.0 2.1 2.2 Wells, A.F. (1984) Structural Inorganic Chemistry. 5th. London, England: Oxford University Press. p.890 ISBN 0-19-855370-6
- ↑ Malmros, Gunnar; Fernholt, Liv; Ballhausen, C. J.; Ragnarsson, Ulf; Rasmussen, S. E.; Sunde, Erling; Sørensen, Nils Andreas (1970). "The Crystal Structure of alpha-Bi2O2". Acta Chemica Scandinavica 24: 384–96. doi:10.3891/acta.chem.scand.24-0384.
- ↑ 4.0 4.1 Radaev, S. F.; Simonov, V. I.; Kargin, Yu. F. (1992). "Structural features of γ-phase Bi2O3 and its place in the sillenite family". Acta Crystallographica Section B 48 (5): 604–9. doi:10.1107/S0108768192003847.
- ↑ Harwig, H. A. (1978). "On the Structure of Bismuthsesquioxide: The α, β, γ, and δ-phase". Zeitschrift für anorganische und allgemeine Chemie 444: 151–66. doi:10.1002/zaac.19784440118.
- ↑ Sillén, Lars Gunnar (1937). "X-Ray Studies on Bismuth Trioxide". Arkiv för kemi, mineralogi och geologi 12A (1). OCLC 73018207.
- ↑ Gattow, G.; Schröder, H. (1962). "Über Wismutoxide. III. Die Kristallstruktur der Hochtemperaturmodifikation von Wismut(III)-oxid (δ-Bi2O3)" (in de). Zeitschrift für anorganische und allgemeine Chemie 318 (3–4): 176–89. doi:10.1002/zaac.19623180307.
- ↑ Willis, B. T. M. (1965). "The anomalous behaviour of the neutron reflexion of fluorite". Acta Crystallographica 18: 75–6. doi:10.1107/S0365110X65000130.
- ↑ Shuk, P; Wiemhöfer, H.-D.; Guth, U.; Göpel, W.; Greenblatt, M. (1996). "Oxide ion conducting solid electrolytes based on Bi2O3". Solid State Ionics 89 (3–4): 179–96. doi:10.1016/0167-2738(96)00348-7.
- ↑ Sammes, N; Tompsett, G.A; Cai, Zhihong (1999). "The chemical reaction between ceria and fully stabilised zirconia". Solid State Ionics 121–5 (1–4): 121–5. doi:10.1016/S0167-2738(98)00538-4.
- ↑ Hirano, Masanori; Oda, Takayuki; Ukai, Kenji; Mizutani; Yasunobu (2003). "Effect of Bi2O3 additives in Sc stabilized zirconia electrolyte on a stability of crystal phase and electrolyte properties". Solid State Ionics 158 (3–4): 215–23. doi:10.1016/S0167-2738(02)00912-8.
- ↑ 12.0 12.1 12.2 Ortiz-Quiñonez, Jose; Zumeta-Dubé, Inti; Díaz, David; Nava-Etzana, Noel; Cruz-Zaragoza, Epifanio (2017). "Bismuth Oxide Nanoparticles Partially Substituted with EuIII, MnIV, and SiIV: Structural, Spectroscopic, and Optical Findings". Inorganic Chemistry 56 (6): 3394–3403. doi:10.1021/acs.inorgchem.6b02923. PMID 28252972.
- ↑ Brauer, Georg (1963), Handbook of Preparative Inorganic Chemistry, 1 (2nd ed.), New York: Academic Press Inc., p. 628
- ↑ Hutcheson, C; Seale, N. S.; McWhorter, A; Kerins, C; Wright, J (2012). "Multi-surface composite vs stainless steel crown restorations after mineral trioxide aggregate pulpotomy: A randomized controlled trial". Pediatric Dentistry 34 (7): 460–7. PMID 23265162.
- ↑ Camilleri, Josette (2014). "Color Stability of White Mineral Trioxide Aggregate in Contact with Hypochlorite Solution". Journal of Endodontics 40 (3): 436–40. doi:10.1016/j.joen.2013.09.040. PMID 24565667.
- ↑ Zhai, Huatian; Fan, Desong; Li, Qiang (September 2022). "Scalable and paint-format colored coatings for passive radiative cooling". Solar Energy Materials and Solar Cells 245: 111853. doi:10.1016/j.solmat.2022.111853. https://www.sciencedirect.com/science/article/abs/pii/S0927024822002732.
Further reading
- Shannon, R. D. (1976). "Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides". Acta Crystallographica Section A 32 (5): 751–67. doi:10.1107/S0567739476001551. Bibcode: 1976AcCrA..32..751S.
- Vannier, R.N.; Mairesse, G.; Abraham, F.; Nowogrocki, G. (1993). "Incommensurate Superlattice in Mo-Substituted Bi4V2O11". Journal of Solid State Chemistry 103 (2): 441–6. doi:10.1006/jssc.1993.1120. Bibcode: 1993JSSCh.103..441V.
 |

