Chemistry:Calcium monophosphide
From HandWiki
| |
| Names | |
|---|---|
| Other names
Calcium phosphide
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
| |
| |
| Properties | |
| CaP (Ca2P2) | |
| Appearance | black solid |
| decomposes | |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H260, H300, H311, H318, H330, H400 | |
| P223, P231+232, P260, P264, P270, P271, P273, P280, P284, P301+310, P302+352, P304+340, P305+351+338, P310, P312, P320, P321, P322, P330, P335+334, P361, P363, P370+378, P391, P402+404 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
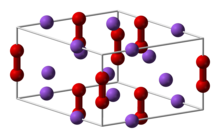
Calcium monophosphide is the inorganic compound with the formula CaP. It is sometimes also known as "calcium phosphide", which also describes a different compound with composition Ca3P2. Calcium monophosphide is a black solid.
Structure and properties
The structures of CaP and sodium peroxide (Na2O2) are very similar.[1] The solid is described as a salt: (Ca2+)2P24−, or Ca2P2. Since the bonding is ionic, the diphosphide centers carry negative charge and are easily protonated. Upon hydrolysis this material releases diphosphine (P2H4):[2]
- Ca2P2 + 4 H2O → 2 Ca(OH)2 + P2H4
The hydrolyses of CaP and calcium carbide (CaC2) are similar, except that diphosphine spontaneously ignites in air. Thus, CaP must be protected from air.
CaP decomposes to Ca3P2 at about 600 °C.
- 3 CaP → Ca3P2 + 1/4 P4
References
- ↑ Iandelli, A. and Franceschi, E., "On the crystal structure of the compounds CaP, SrP, CaAs, SrAs and EuAs", Journal of the Less Common Metals, 1973, volume 30, pp. 211-216. doi:10.1016/0022-5088(73)90107-0
- ↑ Marianne Baudler, Klaus Glinka (1993). "Monocyclic and polycyclic phosphines". Chem. Rev. 93 (4): 1623–1667. doi:10.1021/cr00020a010.
 |

