Chemistry:Calcium sulfide
| |
| Names | |
|---|---|
| IUPAC name
Calcium sulfide
| |
| Other names
Calcium monosulfide,
Hepar calcies, Sulfurated lime Oldhamite | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CaS | |
| Molar mass | 72.143 g/mol |
| Appearance | white crystals hygroscopic |
| Density | 2.59 g/cm3 |
| Melting point | 2,525 °C (4,577 °F; 2,798 K) |
| Hydrolyses | |
| Solubility | Insoluble in alcohol reacts with acid |
Refractive index (nD)
|
2.137 |
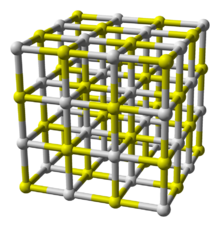
| Structure | |
| Halite (cubic), cF8 | |
| Fm3m, No. 225 | |
a = 569.08 nm[1]
| |
| Octahedral (Ca2+); octahedral (S2−) | |
| Hazards | |
| Main hazards | Reacts with water to release H2S |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H315, H319, H335, H400 | |
| P261, P273, P305+351+338 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Calcium oxide |
Other cations
|
Magnesium sulfide Strontium sulfide Barium sulfide |
Related sulfides
|
Sodium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically has an odour of H2S, which results from small amount of this gas formed by hydrolysis of the salt.
In terms of its atomic structure, CaS crystallizes in the same motif as sodium chloride indicating that the bonding in this material is highly ionic. The high melting point is also consistent with its description as an ionic solid. In the crystal, each S2− ion is surrounded by an octahedron of six Ca2+ ions, and complementarily, each Ca2+ ion surrounded by six S2− ions.
Production
CaS is produced by carbothermic reduction of calcium sulfate, which entails the conversion of carbon, usually as charcoal, to carbon dioxide:
- CaSO4 + 2 C → CaS + 2 CO2
and can react further:
- 3 CaSO4 + CaS → 4 CaO + 4 SO2
In the second reaction the sulfate (+6 oxidation state) oxidizes the sulfide (−2 oxidation state) to sulfur dioxide (+4 oxidation state), while it is being reduced to sulfur dioxide itself (+4 oxidation state).
CaS is also a byproduct in the Leblanc process, a once major industrial process for producing sodium carbonate. In that process sodium sulfide reacts with calcium carbonate:[2]
- Na2S + CaCO3 → CaS + Na2CO3
Millions of tons of this calcium sulfide byproduct was discarded, causing extensive pollution and controversy.[3]
Milk of lime, Ca(OH)2, reacts with elemental sulfur to give a "lime-sulfur", which has been used as an insecticide. The active ingredient is probably a calcium polysulfide, not CaS.[4]
Reactivity and uses
Calcium sulfide decomposes upon contact with water, including moist air, giving a mixture of Ca(SH)2, Ca(OH)2, and Ca(SH)(OH).
- CaS + H2O → Ca(SH)(OH)
- Ca(SH)(OH) + H2O → Ca(OH)2 + H2S
It reacts with acids such as hydrochloric acid to release toxic hydrogen sulfide gas.
- CaS + 2 HCl → CaCl2 + H2S
Calcium sulfide is phosphorescent, and will glow a blood red for up to an hour after a light source is removed.[5]
As a noxious byproduct of the Leblanc process, it can be converted to calcium carbonate and hydrogen sulfide, the latter of which can be used as a sulfur source for the lead chamber process to produce the sulfuric acid necessary for the Leblanc process:
- CaS(s) + CO
2(g) + H
2O(l) → CaCO
3(s) + H
2S(g)
Natural occurrence
Oldhamite is the name for mineralogical form of CaS. It is a rare component of some meteorites and has scientific importance in solar nebula research.[6][7] Burning of coal dumps can also produce the compound.[8]
See also
References
- ↑ "ChemicalBook". https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3391370.htm.
- ↑ Christian Thieme (2000). "Sodium Carbonates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_299. ISBN 978-3527306732.
- ↑ Kiefer, David M. (January 2002). "It was all about alkali". Today's Chemist at Work 11 (1): 45–6. http://pubs.acs.org/subscribe/journals/tcaw/11/i01/html/01chemchron.html.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ "Red Glow in the Dark Powder - Calcium Sulfide". https://www.technoglowproducts.com/red-glow-in-the-dark-powder-calcium-sulfide/.
- ↑ "Oldhamite". https://www.mindat.org/min-2970.html.
- ↑ "List of Minerals". 21 March 2011. https://www.ima-mineralogy.org/Minlist.htm.
- ↑ Kruszewski, Ł. (January 2006). "Oldhamite-periclase-portlandite-fluorite assemblage and coexisting minerals of burnt dump in Siemianowice Ślaskie-Dabrówka Wielka area (Upper Silesia, Poland) - preliminary report". Mineralogia Polonica - Special Papers 28: 118–120. https://www.researchgate.net/publication/285329328.
 |

