Chemistry:Tricalcium phosphate
| |
| |
| Names | |
|---|---|
| IUPAC name
Calcium phosphate
| |
| Other names
Tribasic calcium phosphate, tricalcium bis(phosphate)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
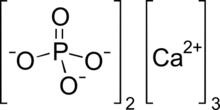
| Ca3(PO4)2 | |
| Molar mass | 310.18 g/mol |
| Appearance | White amorphous powder |
| Density | 3.14 g/cm3[1] |
| Melting point | 1,670 °C (3,040 °F; 1,940 K)[1] |
| 1.2 mg/kg[1] | |
Solubility product (Ksp)
|
2.07×10−33[2] |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−4126 kJ/mol (α-form)[3] |
| Pharmacology | |
| 1=ATC code }} | A12AA01 (WHO) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Calcium pyrophosphate |
Other cations
|
Trimagnesium phosphate Trisodium phosphate Tripotassium phosphate |
Related compounds
|
Monocalcium phosphate Dicalcium phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tricalcium phosphate (sometimes abbreviated TCP), more commonly known as Calcium phosphate, is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.[4][5]
It exists as three crystalline polymorphs α, α′, and β. The α and α′ states are stable at high temperatures.
Nomenclature
Calcium phosphate refers to numerous materials consisting of calcium ions (Ca2+) together with orthophosphates (PO3−4), metaphosphates or pyrophosphates (P2O4−7) and occasionally oxide and hydroxide ions. Especially, the common mineral apatite has formula Ca5(PO4)3X, where X is F, Cl, OH, or a mixture; it is hydroxyapatite if the extra ion is mainly hydroxide. Much of the "tricalcium phosphate" on the market is actually powdered hydroxyapatite.[5]
Preparation
Tricalcium phosphate is produced commercially by treating hydroxyapatite with phosphoric acid and slaked lime.[4]
It cannot be precipitated directly from aqueous solution. Typically double decomposition reactions are employed, involving a soluble phosphate and calcium salts, e.g. (NH4)2HPO4 + Ca(NO3)2.[6] is performed under carefully controlled pH conditions. The precipitate will either be "amorphous tricalcium phosphate", ATCP, or calcium deficient hydroxyapatite, CDHA, Ca9(HPO4)(PO4)5(OH), (note CDHA is sometimes termed apatitic calcium triphosphate).[6][7][8] Crystalline tricalcium phosphate can be obtained by calcining the precipitate. β-Ca3(PO4)2 is generally formed, higher temperatures are required to produce α-Ca3(PO4)2.
An alternative to the wet procedure entails heating a mixture of a calcium pyrophosphate and calcium carbonate:[7]
- CaCO3 + Ca2P2O7 → Ca3(PO4)2 + CO2
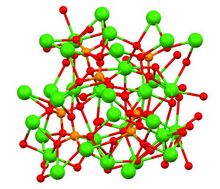
Structure of β-, α- and α′- Ca3(PO4)2 polymorphs
Tricalcium phosphate has three recognised polymorphs, the rhombohedral β form (shown above), and two high temperature forms, monoclinic α and hexagonal α′. β-Tricalcium phosphate has a crystallographic density of 3.066 g cm−3 while the high temperature forms are less dense, α-tricalcium phosphate has a density of 2.866 g cm−3 and α′-tricalcium phosphate has a density of 2.702 g cm−3 All forms have complex structures consisting of tetrahedral phosphate centers linked through oxygen to the calcium ions.[9] The high temperature forms each have two types of columns, one containing only calcium ions and the other both calcium and phosphate.[10]
There are differences in chemical and biological properties between the β and α forms, the α form is more soluble and biodegradable. Both forms are available commercially and are present in formulations used in medical and dental applications.[10]
Occurrence
Calcium phosphate is one of the main combustion products of bone (see bone ash). Calcium phosphate is also commonly derived from inorganic sources such as mineral rock.[11] Tricalcium phosphate occurs naturally in several forms, including:
- as a rock in Morocco, Israel, Philippines , Egypt, and Kola (Russia ) and in smaller quantities in some other countries. The natural form is not completely pure, and there are some other components like sand and lime which can change the composition. The content of P2O5 in most calcium phosphate rocks is 30% to 40% P2O5 by weight.
- in the skeletons and teeth of vertebrate animals
- in milk.
Biphasic calcium phosphate, BCP
Biphasic calcium phosphate, BCP, was originally reported as tricalcium phosphate, but X-Ray diffraction techniques showed that the material was an intimate mixture of two phases, hydroxyapatite (HA) and β-tricalcium phosphate.[12] It is a ceramic.[13] Preparation involves sintering, causing irreversible decomposition of calcium deficient apatites[7] alternatively termed non-stoichiometric apatites or basic calcium phosphate.[14] An example is:[15]
- Ca10−δ(PO4)6−δ(HPO4)δ(OH)2−δ → (1−δ) Ca10(PO4)6(OH)2 + 3δ Ca3(PO4)2
β-TCP can contain impurities, for example calcium pyrophosphate, Ca
2P
2O
7 and apatite. β-TCP is bioresorbable. The biodegradation of BCP involves faster dissolution of the β-TCP phase followed by elimination of HA crystals. β-TCP does not dissolve in body fluids at physiological pH levels, dissolution requires cell activity producing acidic pH.[7]
Uses
Food additive
Tricalcium phosphate is used in powdered spices as an anticaking agent, e.g. to prevent table salt from caking. The calcium phosphates have been assigned European food additive number E341.
Health and beauty products
It is also found in baby powder, antacids and toothpaste.[4] Toothpastes containing functionalized β-tricalcium phosphate (fTCP) may assist with the remineralization of tooth enamel.[16][17][18]
Biomedical
It is also used as a nutritional supplement[19] and occurs naturally in cow milk,[citation needed] although the most common and economical forms for supplementation are calcium carbonate (which should be taken with food) and calcium citrate (which can be taken without food).[20] There is some debate about the different bioavailabilities of the different calcium salts.
It can be used as a tissue replacement for repairing bony defects when autogenous bone graft is not feasible or possible.[21][22][23] It may be used alone or in combination with a biodegradable, resorbable polymer such as polyglycolic acid.[24] It may also be combined with autologous materials for a bone graft.[25][26]
Porous β-tricalcium phosphate scaffolds are employed as drug carrier systems for local drug delivery in bone.[27]
Natural occurrence
Tuite, a natural analogue of tricalcium orthophosphate(V), is a rare component of some meteorites. Its formation is related to shock metamorphism.[28]
References
- ↑ 1.0 1.1 1.2 Template:RubberBible97th
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 978-1-138-56163-2.
- ↑ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed.. Houghton Mifflin Company. p. A21. ISBN 978-0-618-94690-7.
- ↑ 4.0 4.1 4.2 Klaus Schrödter; Gerhard Bettermann; Thomas Staffel; Friedrich Wahl; Thomas Klein; Thomas Hofmann (2012). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3.
- ↑ 5.0 5.1 El Moussaoui, Youssef; Terrisse, Hélène; Quillard, Sophie; Ropers, Marie-Hélène; Humbert, Bernard (January 2023). "The True Nature of Tricalcium Phosphate Used as Food Additive (E341(iii))" (in en). Nanomaterials 13 (12): 1823. doi:10.3390/nano13121823. ISSN 2079-4991. PMID 37368253.
- ↑ 6.0 6.1 Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. (2003). "Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate". Materials Chemistry and Physics 80 (1): 269–277. doi:10.1016/S0254-0584(02)00466-2.
- ↑ 7.0 7.1 7.2 7.3 Rey, C.; Combes, C.; Drouet, C.; Grossin, D. (2011). "1.111 – Bioactive Ceramics: Physical Chemistry". in Ducheyne, Paul. Comprehensive Biomaterials. 1. Elsevier. pp. 187–281. doi:10.1016/B978-0-08-055294-1.00178-1. ISBN 978-0-08-055294-1.
- ↑ Dorozhkin, Sergey V. (December 2012). "Amorphous calcium (ortho)phosphates". Acta Biomaterialia 6 (12): 4457–4475. doi:10.1016/j.actbio.2010.06.031. PMID 20609395.
- ↑ Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. (2003). "Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction". RNAl of Solid State Chemistry 175 (2): 272–277. doi:10.1016/S0022-4596(03)00279-2. Bibcode: 2003JSSCh.175..272Y.
- ↑ 10.0 10.1 Carrodeguas, R.G.; De Aza, S. (2011). "α-Tricalcium phosphate: Synthesis, properties and biomedical applications". Acta Biomaterialia 7 (10): 3536–3546. doi:10.1016/j.actbio.2011.06.019. PMID 21712105.
- ↑ Yacoubou, Jeanne, MS. Vegetarian Journal's Guide To Food Ingredients "Guide to Food Ingredients". The Vegetarian Resource Group, n.d. Web. 14 Sept. 2012.
- ↑ Daculsi, G.; Legeros, R. (2008). "17 – Tricalcium phosphate / hydroxyapatite biphasic ceramics". in Kokubo, Tadashi. Bioceramics and their Clinical Applications. Woodhead Publishing. pp. 395–423. doi:10.1533/9781845694227.2.395. ISBN 978-1-84569-204-9.
- ↑ Salinas, Antonio J.; Vallet-Regi, Maria (2013). "Bioactive ceramics: from bone grafts to tissue engineering". RSC Advances 3 (28): 11116–11131. doi:10.1039/C3RA00166K. Bibcode: 2013RSCAd...311116S.
- ↑ Elliott, J.C. (1994). "3 – Hydroxyapatite and Nonstoichiometric Apatites". Studies in Inorganic Chemistry. 18. Elsevier. pp. 111–189. doi:10.1016/B978-0-444-81582-8.50008-0. ISBN 978-0-444-81582-8.
- ↑ Vallet-Regí, M.; Rodríguez-Lorenzo, L.M. (November 1997). "Synthesis and characterisation of calcium deficient apatite". Solid State Ionics 101–103, Part 2: 1279–1285. doi:10.1016/S0167-2738(97)00213-0.
- ↑ "Remineralization of enamel subsurface lesions using toothpaste containing tricalcium phosphate and fluoride: an in vitro µCT analysis". BMC Oral Health 20 (1): 292. October 2020. doi:10.1186/s12903-020-01286-1. PMID 33109184.
- ↑ "Overview of Calcium Phosphates used in Biomimetic Oral Care". The Open Dentistry Journal 12: 406–423. 2018. doi:10.2174/1874210601812010406. PMID 29988215.
- ↑ "Improving Oral Health with Fluoride-Free Calcium-Phosphate-Based Biomimetic Toothpastes: An Update of the Clinical Evidence". Biomimetics 8 (4): 331. July 2023. doi:10.3390/biomimetics8040331. PMID 37622936.
- ↑ "Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial". J. Clin. Invest. 99 (6): 1287–1294. March 1997. doi:10.1172/JCI119287. PMID 9077538.
- ↑ Straub DA (June 2007). "Calcium supplementation in clinical practice: a review of forms, doses, and indications". Nutr Clin Pract 22 (3): 286–296. doi:10.1177/0115426507022003286. PMID 17507729.
- ↑ "Major bone defect treatment with an osteoconductive bone substitute". Musculoskelet Surg 93 (2): 89–96. September 2009. doi:10.1007/s12306-009-0028-0. PMID 19711008.
- ↑ "The evaluation of a biphasic calcium phosphate ceramic for use in grafting long-bone diaphyseal defects". Journal of Orthopaedic Research 5 (3): 356–365. 1987. doi:10.1002/jor.1100050307. PMID 3040949.
- ↑ "Granular tricalcium phosphate in large cancellous defects". Annals of Clinical and Laboratory Science 16 (6): 467–472. 1986. PMID 3541772.
- ↑ "A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering". Bone 46 (2): 386–395. September 2009. doi:10.1016/j.bone.2009.09.031. PMID 19800045.
- ↑ "Potential of an ultraporous β-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft". European Spine Journal 10 (Suppl 2): S141–S146. October 2001. doi:10.1007/s005860100287. PMID 11716011.
- ↑ "Evaluation of hydroxyapatite and beta-tricalcium phosphate mixed with bone marrow aspirate as a bone graft substitute for posterolateral spinal fusion". Indian Journal of Orthopaedics 43 (3): 234–239. July 2009. doi:10.4103/0019-5413.49387. PMID 19838344.
- ↑ Kundu, B; Lemos A; Soundrapandian C; Sen PS; Datta S; Ferreira JMF; Basu D (2010). "Development of porous HAp and β-TCP scaffolds by starch consolidation with foaming method and drug-chitosan bilayered scaffold based drug delivery system". J. Mater. Sci. Mater. Med. 21 (11): 2955–2969. doi:10.1007/s10856-010-4127-0. PMID 20644982.
- ↑ Tuite. Mindat.org
 |


