Chemistry:Calcium bromide
| |
| Names | |
|---|---|
| IUPAC name
Calcium bromide
| |
| Other names
Calcium dibromide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| CaBr2 | |
| Molar mass | 199.89 g/mol (anhydrous) 235.98 g/mol (dihydrate) |
| Appearance | anhydrous is hygroscopic colorless crystals sharp saline taste |
| Density | 3.353 g/cm3 |
| Melting point | 730 °C (1,350 °F; 1,000 K) |
| Boiling point | 1,815 °C (3,299 °F; 2,088 K) (anhydrous) 810 °C (dihydrate) |
| 125 g/100 mL (0 °C) 143 g/100 mL (20 °C) 312 g/100 mL (100 °C) | |
| Solubility in alcohol, acetone | soluble |
| Acidity (pKa) | 9 |
| -73.8·10−6 cm3/mol | |
| Structure | |
| rhomboid | |
| Thermochemistry | |
Heat capacity (C)
|
75 J/mol K |
Std molar
entropy (S |
130 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
-647.9 kJ/mol |
Gibbs free energy (ΔfG˚)
|
-656.1 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4100 mg/kg (rat, oral) 1580 mg/kg (mouse, subcutaneous) |
| Related compounds | |
Other anions
|
Calcium fluoride Calcium chloride Calcium iodide |
Other cations
|
Beryllium bromide Magnesium bromide Strontium bromide Barium bromide Radium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium bromide is the name for compounds with the chemical formula CaBr2(H2O)x. Individual compounds include the anhydrous material (x = 0), the hexahydrate (x = 6), and the rare dihydrate (x = 2). All are white powders that dissolve in water, and from these solutions crystallizes the hexahydrate. The hydrated form is mainly used in some drilling fluids.[1]
Synthesis, structure, and reactions
It is produced by the reaction of calcium oxide, calcium carbonate with hydrobromic acid or the reaction of calcium metal with elemental bromine.[1]
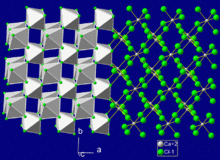
It adopts the rutile structure, featuring octahedral Ca centres bound to six bromide anions, which also bridge to other Ca centres.
When strongly heated in air, calcium bromide will react with oxygen to produce calcium oxide and bromine:
- 2 CaBr2 + O2 → 2 CaO + 2 Br2
In this reaction, the oxygen oxidizes the bromide to bromine.
Uses
It is mainly used as dense aqueous solutions for drilling fluids.[1] It is also used in neuroses medication, freezing mixtures, food preservatives, photography and fire retardants.[2]
Calcium bromide has been shown to undergo complexation with triphenylphosphine oxide, allowing for removal of triphenylphosphine oxide from reaction mixtures without the use of chromatography.[3]
References
- ↑ 1.0 1.1 1.2 Michael J. Dagani, Henry J. Barda, Theodore J. Benya, David C. Sanders “Bromine Compounds” Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_405
- ↑ "Chemical Land 21". http://www.chemicalland21.com/industrialchem/inorganic/CALCIUM%20BROMIDE.htm.
- ↑ Rodríguez Hergueta, Antonio (2022). "Easy Removal of Triphenylphosphine Oxide from Reaction Mixtures by Precipitation with CaBr2". Organic Process Research & Development 26 (6): 1845–1853. doi:10.1021/acs.oprd.2c00104.
External links
 |

