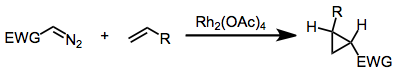Chemistry:Cyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane ((CH
2)
3) rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroid insecticides and a number of quinolone antibiotics (ciprofloxacin, sparfloxacin, etc.). However, the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions.[1] Many of the reactions proceed in a cheletropic manner.
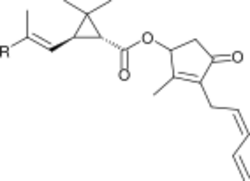
3 and pyrethrin II, R = CO
2CH
3.
Approaches
From alkenes using carbenoid reagents
Several methods exist for converting alkenes to cyclopropane rings using carbene type reagents. As carbenes themselves are highly reactive it is common for them to be used in a stabilised form, referred to as a carbenoid.[2]
Simmons–Smith reaction
In the Simmons–Smith reaction the reactive carbenoid is iodomethylzinc iodide, which is typically formed by a reaction between diiodomethane and a zinc-copper couple. Modifications involving cheaper alternatives have been developed, such as dibromomethane[3] or diazomethane and zinc iodide.[4] The reactivity of the system can also be increased by exchanging the zinc‑copper couple for diethylzinc.[5] Asymmetric versions are known.[6]
Using diazo compounds
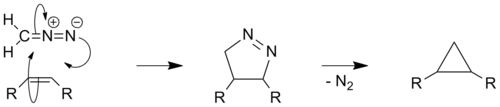
Certain diazo compounds, such as diazomethane, can react with olefins to produce cyclopropanes in a 2 step manner. The first step involves a 1,3-dipolar cycloaddition to form a pyrazoline which then undergoes denitrogenation, either photochemically or by thermal decomposition, to give cyclopropane. The thermal route, which often uses KOH and platinum as catalysts, is also known as the Kishner cyclopropane synthesis after the Russian chemist Nikolai Kischner[7][8] and can also be performed using hydrazine and α,β-unsaturated carbonyl compounds.[9] The mechanism of decomposition has been the subject of several studies and remains somewhat controversial, although it is broadly thought to proceed via a diradical species.[10][11] In terms of green chemistry this method is superior to other carbene based cyclopropanations; as it does not involve metals or halogenated reagents, and produces only N2 as a by-product. However the reaction can be dangerous as trace amounts of unreacted diazo compounds may explode during the thermal rearrangement of the pyrazoline.
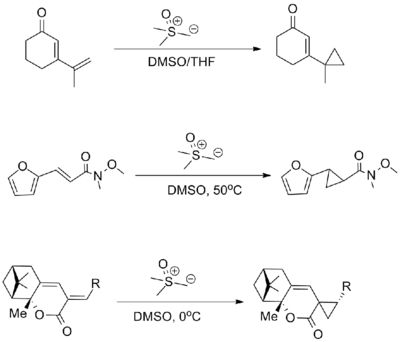
Using diazo compounds with metal catalysis
Methyl phenyldiazoacetate and many related diazo derivatives are precursors to donor-acceptor carbenes, which can be used for cyclopropanation or to insert into C-H bonds of organic substrates. These reactions are catalyzed by dirhodium tetraacetate or, more spectacularly, related chiral derivatives.[12] [13][14]
Using free carbenes
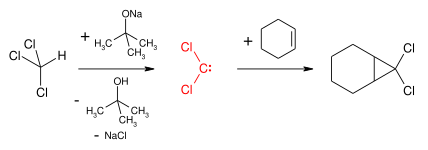
Free carbenes can be employed for cyclopropanation reactions, however there is limited scope for this as few can be produced conveniently and nearly all are unstable (see: carbene dimerization). An exception are dihalocarbenes such as dichlorocarbene or difluorocarbene, which are reasonably stable and will react to form geminal dihalo-cyclopropanes.[15] These compounds can then be used to form allenes via the Skattebøl rearrangement.
The Buchner ring expansion reaction also involves the formation of a stabilised carbene. Cyclopropanation is also stereospecific as the addition of carbene and carbenoids to alkenes is a form of a cheletropic reaction, with the addition taking place in a syn manner. For example, dibromocarbene and cis-2-butene yield cis-2,3-dimethyl-1,1-dibromocyclopropane, whereas the trans isomer exclusively yields the trans cyclopropane.[16]
From alkenes using ylides
Cyclopropanes can be generated using a sulphur ylide in the Johnson–Corey–Chaykovsky reaction,[17] however this process is largely limited to use on electron-poor olefines, particularly α,β-unsaturated carbonyl compounds.
Intramolecular cyclisation
Cyclopropanes can be obtained by a variety of intramolecular cyclisation reactions. A simple method is to use primary haloalkanes bearing appropriately placed electron withdrawing groups. Treatment with a strong base will generate a carbanion which will cyclise in a 3-exo-trig manner, with displacement of the halide. Examples include the formation of cyclopropyl cyanide[18] and cyclopropylacetylene[19] This mechanism also forms the basis of the Favorskii rearrangement.
A related process is the cyclisation of 1,3-dibromopropane via a Wurtz coupling. This was used for the first synthesis of cyclopropane by August Freund in 1881. Originally this reaction was performed using sodium,[20] however the yield can be improved by exchanging this for zinc.[21]
- BrCH2CH2CH2Br + 2 Na → (CH2)3 + 2 NaBr
Other approaches
- The Kulinkovich reaction form cyclopropanols via a reaction between esters and Grignard reagents in presence of a titanium alkoxide.
- The Bingel reaction is a specialised cyclopropanation reaction used to functionalise a fullerene.
- In the di-π-methane rearrangement, photochemical stimulation causes 1,4-dienes to rearrange to form vinylcyclopropanes.[22] These can then undergo vinylcyclopropane rearrangements
- Cyclopropane-fatty-acyl-phospholipid synthase performs cyclopropanation in biological systems
- Using Cobalt(II)–porphyrin catalysis, using diazo compounds and olefins.
Biosynthesis
Although cyclopropanes are relatively rare in biochemistry, many cyclopropanation pathways have been identified in nature. The most common pathways involve ring closure reactions of carbocations in terpenoids. Cyclopropane fatty acids are derived from the attack of S-adenosylmethionine (SAM) on unsaturated fatty acids. The precursor to the hormone ethylene, 1-aminocyclopropane-1-carboxylic acid, is derived directly from SMM via intramolecular nucleophilic displacement of the SMe2 group subsequent to condensation with pyridoxal phosphate.[23] Direct carbene transfer from diazoesters to olefins has also been achieved through in vitro biocatalysis using engineered variants of the cytochrome P450 enzyme from Bacillus megaterium that were optimized by directed evolution.[24]
References
- ↑ Pellissier, Hélène (July 2008). "Recent developments in asymmetric cyclopropanation". Tetrahedron 64 (30–31): 7041–7095. doi:10.1016/j.tet.2008.04.079.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "carbenoids". doi:10.1351/goldbook.C00813.html
- ↑ Fabisch, Bodo; Mitchell, Terence N. (1984). "An inexpensive modification of the Simmons-Smith reaction: The formation of bromomethylzinc bromide as studied by NMR spectroscopy". Journal of Organometallic Chemistry 269 (3): 219–221. doi:10.1016/0022-328X(84)80305-8.
- ↑ Wittig, Georg; Wingler, Frank (1 August 1964). "Über methylenierte Metallhalogenide, IV. Cyclopropan-Bildung aus Olefinen mit Bis-halogenmethyl-zink". Chemische Berichte 97 (8): 2146–2164. doi:10.1002/cber.19640970808.
- ↑ Furukawa, J.; Kawabata, N.; Nishimura, J. (1968). "Synthesis of cyclopropanes by the reaction of olefins with dialkylzinc and methylene iodide". Tetrahedron 24 (1): 53–58. doi:10.1016/0040-4020(68)89007-6.
- ↑ Charette, A. B.; Beauchemin, A. (2001). "Simmons-Smith Cyclopropanation Reaction". Org. React.. 58. p. 1. doi:10.1002/0471264180.or058.01. ISBN 978-0471264187.
- ↑ Lewis, David E. (4 November 2013). "Disability, Despotism, Deoxygenation-From Exile to Academy Member: Nikolai Matveevich Kizhner". Angewandte Chemie International Edition 52 (45): 11704–11712. doi:10.1002/anie.201303165. PMID 24123691.
- ↑ N. M. Kishner, A. Zavadovskii, J. Russ. Phys. Chem. Soc. 43, 1132 (1911).
- ↑ J. Petersen, R.; P. S. Skell, P. (1967). "PHENYLCYCLOPROPANE". Org. Synth. 47: 98. doi:10.15227/orgsyn.047.0098.
- ↑ Crawford, Robert J.; Mishra, Anupama (September 1966). "The Mechanism of the Thermal Decomposition of 1-Pyrazolines and Its Relationship to Cyclopropane Isomerizations". Journal of the American Chemical Society 88 (17): 3963–3969. doi:10.1021/ja00969a014.
- ↑ Muray, Elena; Illa, Ona; Castillo, José A.; Álvarez-Larena, Ángel; Bourdelande, José L.; Branchadell, Vicenç; Ortuño, Rosa M. (June 2003). "Photolysis of Chiral 1-Pyrazolines to Cyclopropanes: Mechanism and Stereospecificity". The Journal of Organic Chemistry 68 (12): 4906–4911. doi:10.1021/jo0342471. PMID 12790598.
- ↑ Davies, H. M. L.; Morton, D. (2011). "Guiding Principles for Site Selective and Stereoselective Intermolecular C–H Functionalization by Donor/Acceptor Rhodium Carbenes". Chemical Society Reviews 40 (4): 1857–1869. doi:10.1039/C0CS00217H. PMID 21359404.
- ↑ Huw M. L. Davies; Wen‐hao Hu; Dong Xing (2015). "Methyl Phenyldiazoacetate". EEROS: 1–10. doi:10.1002/047084289X.rn00444.pub2. ISBN 9780470842898.
- ↑ Lebel, Hélène; Marcoux, Jean-François; Molinaro, Carmela; Charette, André B. (1 April 2003). "Stereoselective Cyclopropanation Reactions". Chemical Reviews 103 (4): 977–1050. doi:10.1021/cr010007e. PMID 12683775.
- ↑ Fedoryński, Michał (1 April 2003). "Syntheses of Dihalocyclopropanes and Their Use in Organic Synthesis". Chemical Reviews 103 (4): 1099–1132. doi:10.1021/cr0100087. PMID 12683778.
- ↑ Skell, P.S.; Garner, A.Y. (1956). "The Stereochemistry of Carbene-Olefin Reactions. Reactions of Dibromocarbene with the cis- and trans-2-Butenes". Journal of the American Chemical Society 78 (14): 3409–3411. doi:10.1021/ja01595a040.
- ↑ Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. (1997). "Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement". Chemical Reviews 97 (6): 2341–2372. doi:10.1021/cr960411r. PMID 11848902.
- ↑ Schlatter, M. J. (1943). "Cyclopropyl Cyanide". Organic Syntheses 23: 20. doi:10.15227/orgsyn.023.0020. http://www.orgsyn.org/demo.aspx?prep=CV3P0223.; Collective Volume, 3, pp. 223.
- ↑ Huntington, Martha; Corley, Edward G.; Thompson, Andrew S. (2000). "Cyclopropylacetylene". Organic Syntheses 77: 231. doi:10.15227/orgsyn.077.0231. http://www.orgsyn.org/demo.aspx?prep=V77P0231.
- ↑ Freund, August (1881). "Über Trimethylen" (in de). Journal für Praktische Chemie 26 (1): 625–635. doi:10.1002/prac.18820260125. https://zenodo.org/record/1427896.
- ↑ Gustavson, G. (1887). "Ueber eine neue Darstellungsmethode des Trimethylens" (in de). J. Prakt. Chem. 36: 300–305. doi:10.1002/prac.18870360127. http://gallica.bnf.fr/ark:/12148/bpt6k90799n/f308.table.
- ↑ IUPAC Gold book definition[yes|permanent dead link|dead link}}]
- ↑ Wessjohann, Ludger A.; Brandt, Wolfgang; Thiemann, Thies (April 2003). "Biosynthesis and Metabolism of Cyclopropane Rings in Natural Compounds". Chemical Reviews 103 (4): 1625–1648. doi:10.1021/cr0100188. PMID 12683792.
- ↑ Coelho, P. S.; Brustad, E. M.; Kannan, A.; Arnold, F. H. (20 December 2012). "Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes". Science 339 (6117): 307–310. doi:10.1126/science.1231434. PMID 23258409. Bibcode: 2013Sci...339..307C. https://authors.library.caltech.edu/36827/7/Coelho.SM.pdf.
 |