Chemistry:Ciprofloxacin
 structure | |
 3D model of ciprofloxacin zwitterion | |
| Clinical data | |
|---|---|
| Trade names | Ciloxan, Cipro, Neofloxin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, topical (ear drops, eye drops) |
| Drug class | Fluoroquinolone |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 70%[2] |
| Protein binding | 30%[2] |
| Metabolism | Liver (incl. CYP1A2) |
| Elimination half-life | 3.5 hours[2] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
| Formula | C17H18FN3O3 |
| Molar mass | 331.347 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections.[3] This includes bone and joint infections, intra-abdominal infections, certain types of infectious diarrhea, respiratory tract infections, skin infections, typhoid fever, and urinary tract infections, among others.[3] For some infections it is used in addition to other antibiotics.[3] It can be taken by mouth, as eye drops, as ear drops, or intravenously.[3][4]
Common side effects include nausea, vomiting, and diarrhea.[3] Severe side effects include an increased risk of tendon rupture, hallucinations, and nerve damage.[3] In people with myasthenia gravis, there is worsening muscle weakness.[3] Rates of side effects appear to be higher than some groups of antibiotics such as cephalosporins but lower than others such as clindamycin.[5] Studies in other animals raise concerns regarding use in pregnancy.[6] No problems were identified, however, in the children of a small number of women who took the medication.[6] It appears to be safe during breastfeeding.[3] It is a second-generation fluoroquinolone with a broad spectrum of activity that usually results in the death of the bacteria.[3][7][8]
Ciprofloxacin was patented in 1980 and introduced in 1987.[9][10] It is on the World Health Organization's List of Essential Medicines.[11][12] The World Health Organization classifies ciprofloxacin as critically important for human medicine.[13] It is available as a generic medication.[3][14] In 2020, it was the 132nd-most-commonly prescribed medication in the United States, with more than 4 million prescriptions.[15][16]
Medical uses
Ciprofloxacin is used to treat a wide variety of infections, including infections of bones and joints, endocarditis, gastroenteritis, malignant otitis externa, respiratory tract infections, cellulitis, urinary tract infections, prostatitis, anthrax, and chancroid.[3]
Ciprofloxacin only treats bacterial infections; it does not treat viral infections such as the common cold. For certain uses including acute sinusitis, lower respiratory tract infections and uncomplicated gonorrhea, ciprofloxacin is not considered a first-line agent.
Ciprofloxacin occupies an important role in treatment guidelines issued by major medical societies for the treatment of serious infections, especially those likely to be caused by Gram-negative bacteria, including Pseudomonas aeruginosa. For example, ciprofloxacin in combination with metronidazole is one of several first-line antibiotic regimens recommended by the Infectious Diseases Society of America for the treatment of community-acquired abdominal infections in adults.[17] It also features prominently in treatment guidelines for acute pyelonephritis, complicated or hospital-acquired urinary tract infection, acute or chronic prostatitis,[18] certain types of endocarditis,[19] certain skin infections,[20] and prosthetic joint infections.[21]
In other cases, treatment guidelines are more restrictive, recommending in most cases that older, narrower-spectrum drugs be used as first-line therapy for less severe infections to minimize fluoroquinolone-resistance development. For example, the Infectious Diseases Society of America recommends the use of ciprofloxacin and other fluoroquinolones in urinary tract infections be reserved to cases of proven or expected resistance to narrower-spectrum drugs such as nitrofurantoin or trimethoprim/sulfamethoxazole.[22] The European Association of Urology recommends ciprofloxacin as an alternative regimen for the treatment of uncomplicated urinary tract infections, but cautions that the potential for "adverse events have to be considered".[18]
Although approved by regulatory authorities for the treatment of respiratory infections, ciprofloxacin is not recommended for respiratory infections by most treatment guidelines due in part to its modest activity against the common respiratory pathogen Streptococcus pneumoniae.[23][24][25] "Respiratory quinolones" such as levofloxacin, having greater activity against this pathogen, are recommended as first line agents for the treatment of community-acquired pneumonia in patients with important co-morbidities and in patients requiring hospitalization (Infectious Diseases Society of America 2007). Similarly, ciprofloxacin is not recommended as a first-line treatment for acute sinusitis.[26][27]
Ciprofloxacin is approved for the treatment of gonorrhea in many countries, but this recommendation is widely regarded as obsolete due to resistance development.[28][29][30]
Pregnancy
In the United States, ciprofloxacin is pregnancy category C.[31][1] This category includes drugs for which no adequate and well-controlled studies in human pregnancy exist, and for which animal studies have suggested the potential for harm to the fetus, but potential benefits may warrant use of the drug in pregnant women despite potential risks. An expert review of published data on experiences with ciprofloxacin use during pregnancy by the Teratogen Information System concluded therapeutic doses during pregnancy are unlikely to pose a substantial teratogenic risk (quantity and quality of data=fair), but the data are insufficient to state no risk exists.[32] Exposure to quinolones, including levofloxacin, during the first-trimester is not associated with an increased risk of stillbirths, premature births, birth defects, or low birth weight.[33]
Two small post-marketing epidemiology studies of mostly short-term, first-trimester exposure found that fluoroquinolones did not increase risk of major malformations, spontaneous abortions, premature birth, or low birth weight.[34][35] The label notes, however, that these studies are insufficient to reliably evaluate the definitive safety or risk of less common defects by ciprofloxacin in pregnant women and their developing fetuses.
Breastfeeding
Fluoroquinolones have been reported as present in a mother's milk and thus passed on to the nursing child.[36][37] The U.S. Food and Drug Administration (FDA) recommends that because of the risk of serious adverse reactions (including articular damage) in infants nursing from mothers taking ciprofloxacin, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Children
Oral and intravenous ciprofloxacin are approved by the FDA for use in children for only two indications due to the risk of permanent injury to the musculoskeletal system:
- Inhalational anthrax (postexposure)[38]
- Complicated urinary tract infections and pyelonephritis due to Escherichia coli,[39] but never as first-line agents.
Current[when?] recommendations by the American Academy of Pediatrics note the systemic use of ciprofloxacin in children should be restricted to infections caused by multidrug-resistant pathogens or when no safe or effective alternatives are available.[40]
Spectrum of activity
Its spectrum of activity includes most strains of bacterial pathogens responsible for community-acquired pneumonias, bronchitis, urinary tract infections, and gastroenteritis.[41] Ciprofloxacin is particularly effective against Gram-negative bacteria (such as Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, Proteus mirabilis, and Pseudomonas aeruginosa), but is less effective against Gram-positive bacteria (such as methicillin-sensitive Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis) than newer fluoroquinolones.[42]
Bacterial resistance
As a result of its widespread use to treat minor infections readily treatable with older, narrower-spectrum antibiotics, many bacteria have developed resistance to this drug, leaving it significantly less effective than it would have been otherwise.[43][44]
Resistance to ciprofloxacin and other fluoroquinolones may evolve rapidly, even during a course of treatment. Numerous pathogens, including enterococci, Streptococcus pyogenes , and Klebsiella pneumoniae (quinolone-resistant) now exhibit resistance.[45] Widespread veterinary usage of fluoroquinolones, particularly in Europe, has been implicated.[46] Meanwhile, some Burkholderia cepacia, Clostridium innocuum, and Enterococcus faecium strains have developed resistance to ciprofloxacin to varying degrees.[47]
Fluoroquinolones had become the class of antibiotics most commonly prescribed to adults in 2002.[48] Nearly half (42%) of those prescriptions in the U.S. were for conditions not approved by the FDA, such as acute bronchitis, otitis media, and acute upper respiratory tract infection, according to a study supported in part by the Agency for Healthcare Research and Quality.[48] Additionally, they were commonly prescribed for medical conditions that were not even bacterial to begin with, such as viral infections, or those for which no proven benefit existed.
Contraindications
Contraindications include:[49]
- Taking tizanidine at the same time
- Use by those who are hypersensitive to any member of the quinolone class of antimicrobial agents
- Use by those who are diagnosed with myasthenia gravis, as muscle weakness may be exacerbated[50]
Ciprofloxacin is also considered to be contraindicated in children (except for the indications outlined above), in pregnancy, to nursing mothers, and in people with epilepsy or other seizure disorders.
Caution may be required in people with Marfan syndrome or Ehlers-Danlos syndrome.[51]
Adverse effects
Adverse effects can involve the tendons, muscles, joints, nerves, and the central nervous system.[52][53]
Rates of adverse effects appear to be higher than with some groups of antibiotics such as cephalosporins but lower than with others such as clindamycin.[5] Compared to other antibiotics some studies find a higher rate of adverse effects[54][55] while others find no difference.[56]
In clinical trials most of the adverse events were described as mild or moderate in severity, abated soon after the drug was discontinued, and required no treatment.[31] Some adverse effects may be permanent.[52] Ciprofloxacin was stopped because of an adverse event in 1% of people treated with the medication by mouth. The most frequently reported drug-related events, from trials of all formulations, all dosages, all drug-therapy durations, and for all indications, were nausea (2.5%), diarrhea (1.6%), abnormal liver function tests (1.3%), vomiting (1%), and rash (1%). Other adverse events occurred at rates of <1%.[57]
Tendon problems
Ciprofloxacin includes a boxed warning in the United States due to an increased risk of tendinitis and tendon rupture, especially in people who are older than 60 years, people who also use corticosteroids, and people with kidney, lung, or heart transplants.[58] Tendon rupture can occur during therapy or even months after discontinuation of the medication.[59] One study found that fluoroquinolone use was associated with a 1.9-fold increase in tendon problems. The risk increased to 3.2 in those over 60 years of age and to 6.2 in those over the age of 60 who were also taking corticosteroids. Among the 46,766 quinolone users in the study, 38 (0.08%) cases of Achilles tendon rupture were identified.[60]
Cardiac arrhythmia
The fluoroquinolones, including ciprofloxacin, are associated with an increased risk of cardiac toxicity, including QT interval prolongation, torsades de pointes, ventricular arrhythmia, and sudden death.[61][53]
Nervous system
Because Ciprofloxacin is lipophilic, it has the ability to cross the blood–brain barrier.[62] The 2013 FDA label warns of nervous system effects. Ciprofloxacin, like other fluoroquinolones, is known to trigger seizures or lower the seizure threshold, and may cause other central nervous system adverse effects. Headache, dizziness, and insomnia have been reported as occurring fairly commonly in postapproval review articles, along with a much lower incidence of serious CNS adverse effects such as tremors, psychosis, anxiety, hallucinations, paranoia, and suicide attempts, especially at higher doses.[5] Like other fluoroquinolones, it is also known to cause peripheral neuropathy that may be irreversible, such as weakness, burning pain, tingling or numbness.[63]
Cancer
Ciprofloxacin is active in six of eight in vitro assays used as rapid screens for the detection of genotoxic effects, but is not active in in vivo assays of genotoxicity.[31] Long-term carcinogenicity studies in rats and mice resulted in no carcinogenic or tumorigenic effects due to ciprofloxacin at daily oral dose levels up to 250 and 750 mg/kg to rats and mice, respectively (about 1.7 and 2.5 times the highest recommended therapeutic dose based upon mg/m2). Results from photo co-carcinogenicity testing indicate ciprofloxacin does not reduce the time to appearance of UV-induced skin tumors as compared to vehicle control.[31]
Other
The other black box warning is that ciprofloxacin should not be used in people with myasthenia gravis due to possible exacerbation of muscle weakness which may lead to breathing problems resulting in death or ventilator support. Fluoroquinolones are known to block neuromuscular transmission.[31] There are concerns that fluoroquinolones including ciprofloxacin can affect cartilage in young children.[64]
Clostridium difficile-associated diarrhea is a serious adverse effect of ciprofloxacin and other fluoroquinolones; it is unclear whether the risk is higher than with other broad-spectrum antibiotics.[65]
A wide range of rare but potentially fatal adverse effects reported to the U.S. FDA or the subject of case reports includes aortic dissection,[66] toxic epidermal necrolysis, Stevens–Johnson syndrome, low blood pressure, allergic pneumonitis, bone marrow suppression, hepatitis or liver failure, and sensitivity to light.[31][67] The medication should be discontinued if a rash, jaundice, or other sign of hypersensitivity occurs.[31]
Children and the elderly are at a much greater risk of experiencing adverse reactions.[68][69]
Overdose
Overdose of ciprofloxacin may result in reversible renal toxicity. Treatment of overdose includes emptying of the stomach by induced vomiting or gastric lavage, as well as administration of antacids containing magnesium, aluminium, or calcium to reduce drug absorption. Renal function and urinary pH should be monitored. Important support includes adequate hydration and urine acidification if necessary to prevent crystalluria. Hemodialysis or peritoneal dialysis can only remove less than 10% of ciprofloxacin.[70] Ciprofloxacin may be quantified in plasma or serum to monitor for drug accumulation in patients with hepatic dysfunction or to confirm a diagnosis of poisoning in acute overdose victims.[71]
Interactions
Ciprofloxacin interacts with certain foods and several other drugs leading to undesirable increases or decreases in the serum levels or distribution of one or both drugs.
Ciprofloxacin should not be taken with antacids containing magnesium or aluminum, highly buffered drugs (sevelamer, lanthanum carbonate, sucralfate, didanosine), or with supplements containing calcium, iron, or zinc. It should be taken two hours before or six hours after these products. Magnesium or aluminum antacids turn ciprofloxacin into insoluble salts that are not readily absorbed by the intestinal tract, reducing peak serum concentrations by 90% or more, leading to therapeutic failure. Additionally, it should not be taken with dairy products or calcium-fortified juices alone, as peak serum concentration and the area under the serum concentration-time curve can be reduced up to 40%. However, ciprofloxacin may be taken with dairy products or calcium-fortified juices as part of a meal.[70][72][73]
Ciprofloxacin inhibits the drug-metabolizing enzyme CYP1A2 and thereby can reduce the clearance of drugs metabolized by that enzyme. CYP1A2 substrates that exhibit increased serum levels in ciprofloxacin-treated patients include tizanidine, theophylline, caffeine, methylxanthines, clozapine, olanzapine, and ropinirole. Co-administration of ciprofloxacin with the CYP1A2 substrate tizanidine (Zanaflex) is contraindicated due to a 583% increase in the peak serum concentrations of tizanidine when administered with ciprofloxacin as compared to administration of tizanidine alone. Use of ciprofloxacin is cautioned in patients on theophylline due to its narrow therapeutic index. The authors of one review recommended that patients being treated with ciprofloxacin reduce their caffeine intake. Evidence for significant interactions with several other CYP1A2 substrates such as cyclosporine is equivocal or conflicting.[73][74][75]
The Committee on Safety of Medicines and the FDA warn that central nervous system adverse effects, including seizure risk, may be increased when NSAIDs are combined with quinolones.[74][76] The mechanism for this interaction may involve a synergistic increased antagonism of GABA neurotransmission.[77][78]
Altered serum levels of the antiepileptic drugs phenytoin and carbamazepine (increased and decreased) have been reported in patients receiving concomitant ciprofloxacin.[74][79][80]
Ciprofloxacin is a potent inhibitor of CYP1A2, CYP2D6, and CYP3A4.[81]
Mechanism of action
Ciprofloxacin is a broad-spectrum antibiotic of the fluoroquinolone class. It is active against some Gram-positive and many Gram-negative bacteria.[82] It functions by inhibiting a type II topoisomerase (DNA gyrase) and topoisomerase IV,[83][84] necessary to separate bacterial DNA, thereby inhibiting cell division. Bacterial DNA fragmentation will occur as a result of inhibition of the enzymes.
Pharmacokinetics
Ciprofloxacin for systemic administration is available as immediate-release tablets, extended-release tablets, an oral suspension, and as a solution for intravenous administration. When administered over one hour as an intravenous infusion,[31] ciprofloxacin rapidly distributes into the tissues, with levels in some tissues exceeding those in the serum. Penetration into the central nervous system is relatively modest, with cerebrospinal fluid levels normally less than 10% of peak serum concentrations. The serum half-life of ciprofloxacin is about 4–6 hours, with 50–70% of an administered dose being excreted in the urine as unmetabolized drug. An additional 10% is excreted in urine as metabolites. Urinary excretion is virtually complete 24 hours after administration. Dose adjustment is required in the elderly and in those with renal impairment.[31]
Ciprofloxacin is weakly bound to serum proteins (20–40%). It is an inhibitor of the drug-metabolizing enzyme cytochrome P450 1A2, which leads to the potential for clinically important drug interactions with drugs metabolized by that enzyme.[85]
Ciprofloxacin is about 70% orally available when administered orally, so a slightly higher dose is needed to achieve the same exposure when switching from IV to oral administration[31]
The extended release oral tablets[86] allow once-daily administration by releasing the drug more slowly in the gastrointestinal tract. These tablets contain 35% of the administered dose in an immediate-release form and 65% in a slow-release matrix. Maximum serum concentrations are achieved between 1 and 4 hours after administration. Compared to the 250- and 500-mg immediate-release tablets, the 500-mg and 1000-mg XR tablets provide higher Cmax, but the 24‑hour AUCs are equivalent.
Ciprofloxacin immediate-release tablets contain ciprofloxacin as the hydrochloride salt, and the XR tablets contain a mixture of the hydrochloride salt as the free base.[87]
Chemical properties
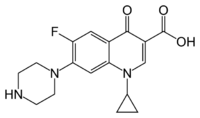
Ciprofloxacin is 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its molecular weight is 331.4 g/mol. It is a faintly yellowish to light yellow crystalline substance.[70]
Ciprofloxacin hydrochloride (USP) is the monohydrochloride monohydrate salt of ciprofloxacin. It is a faintly yellowish to light yellow crystalline substance with a molecular weight of 385.8 g/mol. Its empirical formula is C17H18FN3O3HCl•H2O.[70]
Usage
Ciprofloxacin is the most widely used of the second-generation quinolones.[88][89] In 2010, over 20 million prescriptions were written, making it the 35th-most-commonly prescribed generic drug and the 5th-most-commonly prescribed antibacterial in the U.S.[90]
History
The first members of the quinolone antibacterial class were relatively low-potency drugs such as nalidixic acid, used mainly in the treatment of urinary tract infections owing to their renal excretion and propensity to be concentrated in urine.[91] In 1979, the publication of a patent[92] filed by the pharmaceutical arm of Kyorin Seiyaku Kabushiki Kaisha disclosed the discovery of norfloxacin, and the demonstration that certain structural modifications including the attachment of a fluorine atom to the quinolone ring leads to dramatically enhanced antibacterial potency.[93] In the aftermath of this disclosure, several other pharmaceutical companies initiated research and development programs with the goal of discovering additional antibacterial agents of the fluoroquinolone class.
The fluoroquinolone program at Bayer focused on examining the effects of very minor changes to the norfloxacin structure.[94][95] In 1983, the company published in vitro potency data for ciprofloxacin, a fluoroquinolone antibacterial having a chemical structure differing from that of norfloxacin by the presence of a single carbon atom.[96] This small change led to a two- to 10-fold increase in potency against most strains of Gram-negative bacteria. Importantly, this structural change led to a four-fold improvement in activity against the important Gram-negative pathogen Pseudomonas aeruginosa, making ciprofloxacin one of the most potent known drugs for the treatment of this intrinsically antibiotic-resistant pathogen.
The oral tablet form of ciprofloxacin was approved in October 1987,[97] just one year after the approval of norfloxacin.[98] In 1991, the intravenous formulation was introduced. Ciprofloxacin sales reached a peak of about 2 billion euros in 2001, before Bayer's patent expired in 2004, after which annual sales have averaged around €200 million.[99][100]
The name probably originates from the International Scientific Nomenclature: ci- (alteration of cycl-) + propyl + fluor- + ox- + az- + -mycin.[101]
Society and culture
Cost
It is available as a generic medication and not very expensive. At least one company, Turtle Pharma Private Limited provides industrial-size amounts[3][14]
Generic equivalents
On 24 October 2001, the Prescription Access Litigation (PAL) project filed suit to dissolve an agreement between Bayer and three of its competitors which produced generic versions of drugs (Barr Laboratories, Rugby Laboratories, and Hoechst-Marion-Roussel) that PAL claimed was blocking access to adequate supplies and cheaper, generic versions of ciprofloxacin. The plaintiffs charged that Bayer Corporation, a unit of Bayer AG, had unlawfully paid the three competing companies a total of $200 million to prevent cheaper, generic versions of ciprofloxacin from being brought to the market, as well as manipulating its price and supply. Numerous other consumer advocacy groups joined the lawsuit. On 15 October 2008, five years after Bayer's patent had expired, the United States District Court for the Eastern District of New York granted Bayer's and the other defendants' motion for summary judgment, holding that any anticompetitive effects caused by the settlement agreements between Bayer and its codefendants were within the exclusionary zone of the patent and thus could not be redressed by federal antitrust law,[102] in effect upholding Bayer's agreement with its competitors.
Available forms
Ciprofloxacin for systemic administration is available as immediate-release tablets, as extended-release tablets, as an oral suspension, and as a solution for intravenous infusion. It is also available for local administration as eye drops and ear drops.
Litigation
A class action was filed against Bayer AG on behalf of employees of the Brentwood Post Office in Washington, D.C., and workers at the U.S. Capitol, along with employees of American Media, Inc. in Florida and postal workers in general who alleged they developed serious adverse effects from taking ciprofloxacin in the aftermath of the anthrax attacks in 2001. The action alleged Bayer failed to warn class members of the potential side effects of the drug, thereby violating the Pennsylvania Unfair Trade Practices and Consumer Protection Laws. The class action was defeated and the litigation abandoned by the plaintiffs.[103] A similar action was filed in 2003 in New Jersey by four New Jersey postal workers but was withdrawn for lack of grounds, as workers had been informed of the risks of ciprofloxacin when they were given the option of taking the drug.[104][105]
Research
As resistance to ciprofloxacin has grown since its introduction, research has been conducted to discover and develop analogs that can be effective against resistant bacteria; some have been looked at in antiviral models as well.[106]
See also
- Ciprofloxacin/dexamethasone
References
- ↑ 1.0 1.1 "Ciprofloxacin Use During Pregnancy". 7 January 2019. https://www.drugs.com/pregnancy/ciprofloxacin.html.
- ↑ 2.0 2.1 2.2 "A Review of New Fluoroquinolones: Focus on their Use in Respiratory Tract Infections". Treatments in Respiratory Medicine 5 (6): 437–465. 2006. doi:10.2165/00151829-200605060-00009. PMID 17154673.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 "Ciprofloxacin Hydrochloride". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/ciprofloxacin-hydrochloride.html.
- ↑ "Ciprofloxacin Hcl Drops". 22 February 2018. https://www.webmd.com/drugs/2/drug-91414-6093/ciprofloxacin-ophthalmic-eye/ciprofloxacin-drops-ophthalmic/details.
- ↑ 5.0 5.1 5.2 "The perils of prescribing fluoroquinolones". The Journal of Family Practice 62 (4): 191–197. April 2013. PMID 23570031. http://www.jfponline.com/Pages.asp?AID=11235.
- ↑ 6.0 6.1 "Prescribing medicines in pregnancy database". Government of Australia. 23 August 2015. http://www.tga.gov.au/hp/medicines-pregnancy.htm.
- ↑ "Quinolone generations: natural history or natural selection?". The Journal of Antimicrobial Chemotherapy 46 Suppl T1: 17–24. July 2000. doi:10.1093/oxfordjournals.jac.a020889. PMID 10997595.
- ↑ "Quinolones: a comprehensive review". American Family Physician 65 (3): 455–464. February 2002. doi:10.1016/s0022-5347(17)67120-9. PMID 1185862. http://www.aafp.org/link_out?pmid=11858629.
- ↑ Oxford Handbook of Infectious Diseases and Microbiology. OUP Oxford. 2009. p. 56. ISBN 978-0-19-103962-1. https://books.google.com/books?id=5W-WBQAAQBAJ&pg=PT56.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 500. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA500.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. 2019. ISBN 9789241515528.
- ↑ 14.0 14.1 Tarascon pharmacopoeia (15th ed.). Jones & Bartlett Publishers. 2014. p. 85. ISBN 978-1-284-05671-6. https://books.google.com/books?id=F6YdAwAAQBAJ&pg=PA85.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Ciprofloxacin – Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Ciprofloxacin.
- ↑ "Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America". Clinical Infectious Diseases 50 (2): 133–64. January 2010. doi:10.1086/649554. PMID 20034345.
- ↑ 18.0 18.1 "Guidelines on Urological Infections". European Association of Urology. 2013. http://www.uroweb.org/gls/pdf/18_Urological%20infections_LR.pdf.
- ↑ "Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America". Circulation 111 (23): e394–434. June 2005. doi:10.1161/CIRCULATIONAHA.105.165564. PMID 15956145.
- ↑ "Practice guidelines for the diagnosis and management of skin and soft-tissue infections". Clinical Infectious Diseases 41 (10): 1373–406. November 2005. doi:10.1086/497143. PMID 16231249.
- ↑ "Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America". Clinical Infectious Diseases 56 (1): e1–e25. January 2013. doi:10.1093/cid/cis803. PMID 23223583.
- ↑ "International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases". Clinical Infectious Diseases 52 (5): e103–20. March 2011. doi:10.1093/cid/ciq257. PMID 21292654.
- ↑ "Ciprofloxacin in acute exacerbations of chronic bronchitis". The Journal of Antimicrobial Chemotherapy 18 (3): 407–413. September 1986. doi:10.1093/jac/18.3.407. PMID 3490468.
- ↑ "Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials". Canadian Medical Association Journal 179 (12): 1269–1277. December 2008. doi:10.1503/cmaj.080358. PMID 19047608.
- ↑ "Ciprofloxacin in general practice". BMJ 308 (6941): 1437. May 1994. doi:10.1136/bmj.308.6941.1437. PMID 8019264.
- ↑ "Fluoroquinolones compared with beta-lactam antibiotics for the treatment of acute bacterial sinusitis: a meta-analysis of randomized controlled trials". Canadian Medical Association Journal 178 (7): 845–854. March 2008. doi:10.1503/cmaj.071157. PMID 18362380.
- ↑ "IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults". Clinical Infectious Diseases 54 (8): e72–e112. April 2012. doi:10.1093/cid/cir1043. PMID 22438350.
- ↑ Department of Health and Human Services; Centers for Disease Control and Prevention (November 2004). "Gonococcal Isolate Surveillance Project (GISP) Annual Report – 2003". USA: Center for Disease Controlo. https://www.cdc.gov/STD/gisp2003/GISP2003.pdf.
- ↑ "Ciprofloxacin resistant gonorrhoea: the situation in Scotland and implications for therapy". SCIEH Weekly Report 37. 22 July 2003. ISSN 1357-4493. http://www.documents.hps.scot.nhs.uk/ewr/pdf2003/0329.pdf. Retrieved 30 August 2009.
- ↑ Centers for Disease Control Prevention (CDC) (April 2007). "Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections". MMWR. Morbidity and Mortality Weekly Report 56 (14): 332–336. PMID 17431378. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5614a3.htm.
- ↑ 31.0 31.1 31.2 31.3 31.4 31.5 31.6 31.7 31.8 31.9 "US Cipro label". U.S. Food and Drug Administration (FDA). July 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019537s087,020780s044lbl.pdf. For label updates see FDA Index page for NDA 019537
- ↑ "[Illness, anxiety and the physician. An example from neurology and neurorehabilitation"]. Wiener Medizinische Wochenschrift 141 (22): 512–25. May 1995. PMID 1801454.
- ↑ "Pregnancy Outcomes Following Exposure to Quinolone Antibiotics – a Systematic-Review and Meta-Analysis". Pharm. Res. 35 (5): 109. March 2018. doi:10.1007/s11095-018-2383-8. PMID 29582196.
- ↑ "Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study". Antimicrobial Agents and Chemotherapy 42 (6): 1336–9. June 1998. doi:10.1128/AAC.42.6.1336. PMID 9624471.
- ↑ "Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European Network of Teratology Information Services (ENTIS)". European Journal of Obstetrics, Gynecology, and Reproductive Biology 69 (2): 83–9. November 1996. doi:10.1016/0301-2115(95)02524-3. PMID 8902438.
- ↑ "Fetal and maternal tissue distribution of the new fluoroquinolone DW-116 in pregnant rats". Comparative Biochemistry and Physiology. Toxicology & Pharmacology 136 (1): 95–102. September 2003. doi:10.1016/j.cca.2003.08.004. PMID 14522602.
- ↑ "Penetration of fleroxacin into breast milk and pharmacokinetics in lactating women". Antimicrobial Agents and Chemotherapy 37 (2): 293–6. February 1993. doi:10.1128/AAC.37.2.293. PMID 8452360.
- ↑ "Cipro Labeling Revision Letter 08/30/2000 Supplement 008 New or Modified Indication". U.S. Food and Drug Administration (FDA). 30 August 2000. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2000/20780S08ltr.pdf.
- ↑ "Cipro Labeling Revision Letter 03/25/2004 Supplement 049 Patient Population Altered". U.S. Food and Drug Administration (FDA). 25 March 2004. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/19537s049,19857s031,19847s027,20780s013ltr.pdf.
- ↑ "Systemic use of fluoroquinolone in children". Korean Journal of Pediatrics 56 (5): 196–201. May 2013. doi:10.3345/kjp.2013.56.5.196. PMID 23741232.
- ↑ Pharmcards review cards for medical students (4th ed.). Philadelphia: Wolters Kluwer|Lippincott Williams & Wilkins. 2010. ISBN 978-0-7817-8741-3. OCLC 893525059.[page needed]
- ↑ "Fluoroquinolones – UpToDate". 12 February 2018. https://www.uptodate.com/contents/fluoroquinolones.
- ↑ "Bacterial resistance to ciprofloxacin in Greece: results from the National Electronic Surveillance System. Greek Network for the Surveillance of Antimicrobial Resistance". Emerging Infectious Diseases 5 (3): 471–6. 1999. doi:10.3201/eid0503.990325. PMID 10341191.
- ↑ "Bacterial resistance prompts concern among health officials". Minnesota Department of Health. 26 February 2009. http://www.health.state.mn.us/news/pressrel/2009/bacterial022609.html.
- ↑ M Jacobs, Worldwide Overview of Antimicrobial Resistance. International Symposium on Antimicrobial Agents and Resistance 2005.
- ↑ "Update on Extra-Label Use of Fluoroquinolones" (Press release). Center for Veterinary Medicine (CVM). 16 July 1996. Archived from the original on 9 March 2010. Retrieved 12 August 2009.
- ↑ "Ciprofloxacin Data Sheet". Toku-E. 1 December 2010. http://www.toku-e.com/Upload/Products/PDS/20120618005735.pdf.
- ↑ 48.0 48.1 "Fluoroquinolone prescribing in the United States: 1995 to 2002". The American Journal of Medicine 118 (3): 259–68. March 2005. doi:10.1016/j.amjmed.2004.09.015. PMID 15745724.
- ↑ "Cipro Labeling Revision 02/25/2011 Supplement 075". U.S. Food and Drug Administration (FDA). 25 February 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019537s075,020780s033lbl.pdf.
- ↑ "Ciprofloxacin". StatPearls. Treasure Island, FL: StatPearls Publishing. 2022. http://www.ncbi.nlm.nih.gov/books/NBK535454/. Retrieved 31 January 2022.
- ↑ "Ciprofloxacin accelerates aortic enlargement and promotes dissection and rupture in Marfan mice". The Journal of Thoracic and Cardiovascular Surgery 163 (3): e215–e226. March 2022. doi:10.1016/j.jtcvs.2020.09.069. PMID 34586071.
- ↑ 52.0 52.1 "Drug Safety and Availability – FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects". U.S. Food and Drug Administration (FDA). https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm.
- ↑ 53.0 53.1 "Fluoroquinolones increase the risk of serious arrhythmias: A systematic review and meta-analysis". Medicine (Baltimore) 96 (44): e8273. November 2017. doi:10.1097/MD.0000000000008273. PMID 29095256.
- ↑ "Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection". Antimicrobial Agents and Chemotherapy 57 (5): 2326–32. May 2013. doi:10.1128/AAC.02176-12. PMID 23478961.
- ↑ "Fluoroquinolones vs beta-lactams for empirical treatment of immunocompetent patients with skin and soft tissue infections: a meta-analysis of randomized controlled trials". Mayo Clinic Proceedings 81 (12): 1553–66. December 2006. doi:10.4065/81.12.1553. PMID 17165634.
- ↑ "Comparative effectiveness of antibiotics for uncomplicated urinary tract infections: network meta-analysis of randomized trials". Family Practice 29 (6): 659–70. December 2012. doi:10.1093/fampra/cms029. PMID 22516128.
- ↑ "Cipro IV Meta-analysis". U.S. Food and Drug Administration (FDA). November 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019847s046,019857s053lbl.pdf.
- ↑ "Tendon Injury and Fluoroquinolone Use: A Systematic Review". Drug Saf 36 (9): 709–21. September 2013. doi:10.1007/s40264-013-0089-8. PMID 23888427.
- ↑ "[Rupture of the patellar ligament one month after treatment with fluoroquinolone"] (in fr). Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur 86 (5): 495–7. September 2000. PMID 10970974. http://www.masson.fr/masson/MDOI-RCO-09-2000-86-5-0035-1040-101019-ART7.
- ↑ "Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study". Drug Safety 29 (10): 889–96. 2006. doi:10.2165/00002018-200629100-00006. PMID 16970512.
- ↑ "Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-analysis and Network Meta-analysis". Drug Saf 42 (4): 529–538. October 2018. doi:10.1007/s40264-018-0751-2. PMID 30368737.
- ↑ "SIADH associated with ciprofloxacin". The Annals of Pharmacotherapy 47 (10): 1359–63. October 2013. doi:10.1177/1060028013502457. PMID 24259701.
- ↑ "FDA Drug Safety Communication: FDA requires label changes to warn of risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection". U.S. Food and Drug Administration (FDA). https://www.fda.gov/Drugs/DrugSafety/ucm365050.htm.
- ↑ "Cipro (ciprofloxacin hydrochloride) Tablets". U.S. Food and Drug Administration (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019537s074,020780s032lbl.pdf.
- ↑ "Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence". Current Medical Research and Opinion 24 (2): 329–33. February 2008. doi:10.1185/030079908X253735. PMID 18067688.
- ↑ "Drug Safety and Availability – FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients". U.S. Food and Drug Administration (FDA). https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm.
- ↑ "Risk of hepatotoxicity associated with fluoroquinolones: a national case-control safety study". American Journal of Health-System Pharmacy 71 (1): 37–43. January 2014. doi:10.2146/ajhp130165. PMID 24352180.
- ↑ "The safety profile of moxifloxacin and other fluoroquinolones in special patient populations". Current Medical Research and Opinion 23 (6): 1403–13. June 2007. doi:10.1185/030079907X188099. PMID 17559736.
- ↑ "Antimicrobial safety: focus on fluoroquinolones". Clinical Infectious Diseases 41 (Suppl 2): S144–57. July 2005. doi:10.1086/428055. PMID 15942881.
- ↑ 70.0 70.1 70.2 70.3 "Cipro Labeling Revision 04/06/2009 Supplement 073". U.S. Food and Drug Administration (FDA). 6 April 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019537s073,020780s030lbl.pdf.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 313–315. ISBN:978-0-9626523-7-0.
- ↑ "New oral macrolide and fluoroquinolone antibiotics: an overview of pharmacokinetics, interactions, and safety". Clinical Infectious Diseases 17 (Suppl 1): S192–9. August 1993. doi:10.1093/clinids/17.supplement_1.s192. PMID 8399914.
- ↑ 73.0 73.1 "Pharmacokinetic drug interactions of antimicrobial drugs: a systematic review on oxazolidinones, rifamycines, macrolides, fluoroquinolones, and Beta-lactams". Pharmaceutics 3 (4): 865–913. November 2011. doi:10.3390/pharmaceutics3040865. PMID 24309312.
- ↑ 74.0 74.1 74.2 "Cipro Labeling Revision 10/03/2008 Supplement 068". U.S. Food and Drug Administration (FDA). 3 October 2008. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019537s68,19847s42,19857s49,20780s26,21473s24lbl.pdf.
- ↑ "Drug interactions with quinolones". The Journal of Antimicrobial Chemotherapy 26 Suppl D: 7–29. November 1990. doi:10.1093/jac/26.suppl_D.7. PMID 2286594.
- ↑ Royal Pharmaceutical Society of Great Britain (2009). "5 Infections". British National Formulary (BNF 57). BMJ Group and RPS Publishing. ISBN 978-0-85369-845-6.
- ↑ "Adverse reactions to fluoroquinolones. an overview on mechanistic aspects". Current Medicinal Chemistry 8 (4): 371–84. March 2001. doi:10.2174/0929867013373435. PMID 11172695.
- ↑ "Drug interactions with quinolone antibacterials". Drug Safety 7 (4): 268–81. 1992. doi:10.2165/00002018-199207040-00003. PMID 1524699.
- ↑ "Therapeutic effects of ciprofloxacin on the pharmacokinetics of carbamazepine in healthy adult male volunteers". Pakistan Journal of Pharmaceutical Sciences 24 (1): 63–8. January 2011. PMID 21190921.
- ↑ "Risk of seizures from concomitant use of ciprofloxacin and phenytoin in patients with epilepsy". Canadian Medical Association Journal 158 (1): 104–5, 108–9. January 1998. PMID 9475922.
- ↑ "The pharmacological importance of cytochrome CYP3A4 in the palliation of symptoms: review and recommendations for avoiding adverse drug interactions". Supportive Care in Cancer 15 (3): 251–7. March 2007. doi:10.1007/s00520-006-0127-5. PMID 17139496.
- ↑ First aid for the USMLE step 2 CK (6th ed.). McGraw-Hill Medical. June 2007. ISBN 9780071487955. https://archive.org/details/firstaidcasesfor00taol.
- ↑ "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiology and Molecular Biology Reviews 61 (3): 377–92. September 1997. doi:10.1128/mmbr.61.3.377-392.1997. PMID 9293187.
- ↑ "DNA topoisomerases and their poisoning by anticancer and antibacterial drugs". Chemistry & Biology 17 (5): 421–33. May 2010. doi:10.1016/j.chembiol.2010.04.012. PMID 20534341.
- ↑ Ciprofloxacin Monograph. Accessed 16 December 2021.
- ↑ "Cipro XR Prescribing Information". U.S. Food and Drug Administration (FDA). http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/21554_ciproXR_lbl.pdf.
- ↑ "CIPRO (ciprofloxacin hydrochloride) TABLETS". U.S. Food and Drug Administration (FDA). 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019537s68,19847s42,19857s49,20780s26,21473s24lbl.pdf.
- ↑ "Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries". Clinical Infectious Diseases 44 (8): 1091–5. April 2007. doi:10.1086/512810. PMID 17366456.
- ↑ "British Columbia Annual Summary of Antibiotics Utilization 2010". http://www.bccdc.ca/NR/rdonlyres/BC629780-7E03-4153-B67B-5CFD4F521DAC/0/Full2010AntibioticConsumptionReport_aug2012.pdf.
- ↑ "2010 Top 200 generic drugs by total prescriptions". http://drugtopics.modernmedicine.com/drugtopics/data/articlestandard/drugtopics/252011/727243/article.pdf.
- ↑ "Urinary tract antiseptics". The Medical Clinics of North America 66 (1): 199–208. January 1982. doi:10.1016/s0025-7125(16)31453-5. PMID 7038329.
- ↑ "Patent US4146719 - Piperazinyl derivatives of quinoline carboxylic acids - Google Patents". https://www.google.com/patents/US4146719.
- ↑ "Comparative in vitro activity of norfloxacin (MK-0366) and ten other oral antimicrobial agents against urinary bacterial isolates". Antimicrobial Agents and Chemotherapy 21 (5): 848–51. May 1982. doi:10.1128/AAC.21.5.848. PMID 6213200.
- ↑ "Patent US4547503 – 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[4-(oxo-alkyl)-1-piperazinyl ... – Google Patents". https://www.google.com/patents/US4547503.
- ↑ "Patent US4544658 – 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(alkyl-1-piperazinyl)quinoline-3 ... – Google Patents". https://www.google.com/patents/US4544658?pg=PA1.
- ↑ "In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents". Antimicrobial Agents and Chemotherapy 23 (4): 559–64. April 1983. doi:10.1128/aac.23.4.559. PMID 6222695.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations N019537". U.S. Food and Drug Administration (FDA). http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=019537&TABLE1=OB_Rx.
- ↑ "Orange Book Detail Record Search". U.S. Food and Drug Administration (FDA). http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=019384&TABLE1=OB_Rx.
- ↑ "www.sec.gov". https://www.sec.gov/Archives/edgar/data/1144145/000115697302000306/f00360e20vf.txt.
- ↑ Dan Prochilo for Law360 18 November 2013 Bayer's $74M Cipro Pay-For-Delay Deal Approved In Calif.
- ↑ "Definition of CIPROFLOXACIN". Definition of CIPROFLOXACIN. Meriam-Webster. https://www.merriam-webster.com/dictionary/ciprofloxacin. Retrieved 10 April 2022.
- ↑ United States Court of Appeals for the Federal Circuit (2008). "United States Court of Appeals for the Federal Circuit". USA. http://www.cafc.uscourts.gov/opinions/08-1097.pdf.
- ↑ "Legal Brief of Postal Employees Cases (EEOC, MSPB, District Courts)". USA: Postal Reporter. http://www.lunewsviews.com/legal_briefs_archives.htm#cipro.
- ↑ Los Angeles Times, from wire service reports. 19 October 2003 Postal Workers Sue Over Anthrax Scare Antibiotic
- ↑ Bill Lewis, President of Trenton Metro Area Local, American Postal Workers Union, AFL-CIO. 7 December 2003 Trenton Metro Area Local: Welcome to Bill's Corner Page accessed 23 October 2014
- ↑ "Ciprofloxacin derivatives and their antibacterial activities". European Journal of Medicinal Chemistry 146: 599–612. February 2018. doi:10.1016/j.ejmech.2018.01.078. PMID 29407984.
External links
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |