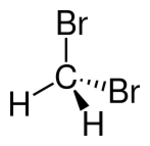
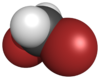
Chemistry:Dibromomethane
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dibromomethane[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|Section1=! colspan=2 style="background: #f8eaba; text-align: center;" |Identifiers
|-
|
|
|-
|
|
|-
|
| 969143 |- | ChEBI
|
|-
|
|-
|
- 200-824-2
|-
|
| 25649 |-
| MeSH
| methylene+bromide
|-
|
|
|- | RTECS number
|
- PA7350000
|- | UNII
|
|- | UN number | 2664 |-
| colspan="2" |
- InChI=1S/CH2Br2/c2-1-3/h1H2
 Key: FJBFPHVGVWTDIP-UHFFFAOYSA-N
Key: FJBFPHVGVWTDIP-UHFFFAOYSA-N
|-
| colspan="2" |
- BrCBr
|- |Section2=! colspan=2 style="background: #f8eaba; text-align: center;" |Properties
|-
|
| CH2Br2
|- | Molar mass
| 173.835 g·mol−1
|- | Appearance | Colorless liquid |-
| Density | 2.477 g⋅mL−1 |- | Melting point | −52.70 °C; −62.86 °F; 220.45 K
|- | Boiling point | 96 to 98 °C; 205 to 208 °F; 369 to 371 K
|-
|
| 12.5 g⋅L−1 (at 20 °C) |-
| Vapor pressure | 4.65 kPa (at 20.0 °C) |-
|
constant (kH)
| 9.3 μmol⋅Pa−1⋅kg−1 |-
|
| −65.10·10−6⋅cm3/mol |-
|
| 1.541 |- |Section3=! colspan=2 style="background: #f8eaba; text-align: center;" |Thermochemistry
|-
|
| 104.1 J⋅K−1⋅mol−1 |- |Section4=! colspan=2 style="background: #f8eaba; text-align: center;" |Hazards
|-
| GHS pictograms
|  |-
| GHS Signal word
|WARNING
|-
| GHS Signal word
|WARNING
|-
|
| H332, H412 |-
|
| P273 |-
| NFPA 704 (fire diamond)
|
|-
| colspan=2 style="text-align:left; background-color:#f1f1f1;" | Lethal dose or concentration (LD, LC):
|-
|- style="background:#f4f4f4;"
| style="padding-left:1em;" |
|
- 1 g⋅kg−1 (oral, rabbit)
- 3.738 g⋅kg−1 (subcutaneous, mouse)
- >4 g⋅kg−1 (dermal, rabbit)
|-
|- |Section5=! colspan=2 style="background: #f8eaba; text-align: center;" |Related compounds
|-
|
|
|- }} Dibromomethane or methylene bromide, or methylene dibromide is a halomethane with the formula CH2Br2. It is slightly soluble in water but very soluble in organic solvents. It is a colorless liquid.
Preparation
Dibromomethane is prepared commercially from dichloromethane via bromochloromethane:
- 6 CH2Cl2 + 3 Br2 + 2 Al → 6 CH2BrCl + 2 AlCl3
- CH2Cl2 + HBr → CH2BrCl + HCl
The latter route requires aluminium trichloride as a catalyst.[2] The bromochloromethane product from either reaction can further react in a similar manner:
- 6 CH2BrCl + 3 Br2 + 2 Al → 6 CH2Br2 + 2 AlCl3
- CH2BrCl + HBr → CH2Br2 + HCl
In the laboratory, it is prepared from bromoform using sodium arsenite and sodium hydroxide:[3]
- CHBr3 + Na3AsO3 + NaOH → CH2Br2 + Na3AsO4 + NaBr
Another way is to prepare it from diiodomethane and bromine.
Uses
Dibromomethane is used as a solvent, gauge fluid, and in organic synthesis (often as 1H-NMR internal standard).[2] It is a convenient agent for converting catechols to their methylenedioxy derivatives.
Natural occurrence
It is naturally produced by marine algae and liberated to the oceans. Releasing on soil causes it to evaporate and leach into the ground. Releasing in water causes it to be lost mainly by volatilisation with a half life of 5.2 hours. It has no significant degradation biological or abiological effects. In the atmosphere it will be lost because of reaction with photochemically produced hydroxyl radicals. The estimated half life of this reaction is 213 days.
References
- Podsiadlo M.; Dziubek K.; Szafranski M.; Katrusiak A. (December 2006). "Molecular interactions in crystalline dibromomethane and diiodomethane, and the stabilities of their high-pressure and low-temperature phases". Acta Crystallogr. B 62 (6): 1090–1098(9). doi:10.1107/S0108768106034963. PMID 17108664. http://scripts.iucr.org/cgi-bin/paper?bk5037. Retrieved 2007-06-29.
- ↑ "methylene bromide - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 25 March 2005. Identification. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3024&loc=ec_rcs#x291.
- ↑ 2.0 2.1 Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
- ↑ W. W. Hartman, E. E. Dreger (1929). "Methylene bromide". Org. Synth. 9: 56. doi:10.15227/orgsyn.009.0056.
External links
 |


