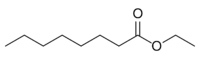
Chemistry:Ethyl octanoate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl octanoate | |
| Other names
Ethyl caprylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C10H20O2 | |
| Molar mass | 172.268 g·mol−1 |
| Density | 0.86215 g/cm3[1] |
| Melting point | −48 °C (−54 °F; 225 K)[4] |
| Boiling point | 208 °C (406 °F; 481 K)[4] |
| 70.1 mg/L [2][3] | |
| Vapor pressure | 0.2 mbar at 20 °C; 3.18 mbar at 60 °C[4] |
| Viscosity | 1.411 mPa·s[1] |
| Hazards | |
| Flash point | 79 °C (174 °F; 352 K)[4] |
| 325 °C (617 °F; 598 K)[4] | |
| Explosive limits | 0.7 - Vol.%[4] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
25.96 g/kg (rat, oral)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethyl octanoate, also known as ethyl caprylate, is a fatty acid ester formed from caprylic acid and ethanol. A colorless liquid at room temperature, it has the semi-developed formula of CH3(CH2)6COOCH2CH3, and is used in food industries as a flavoring and in the perfume industry as a scent additive. It is present in many fruits and alcoholic beverages, and has a strong odor of fruit and flowers. It is used in the creation of synthetic fruity scents.[5]
Synthesis
Ethyl octanoate can be synthesized from caprylic acid and ethanol via a classic Fischer–Speier esterification.
[math]\ce{ CH3(CH2)6COOH + CH3CH2OH <=> CH3(CH2)6COOCH2CH3 + H2O }[/math]
Equilibrium can be shifted towards the right side of the equation through removal of water.
Uses
Ethyl octanoate does not see widespread use due to the greater availability of reasonably similar esters such as ethyl acetate. However, there are certain applications where it fills a niche. Ethyl octanoate has a strong odor of fruit and flowers and a taste of apricot, and as such it can be used as a flavoring or to create scents. It sees some use as a cleaning agent.[6]
Ethyl octanoate also sees incidental use. It is found in some wines, where overall ester concentration and composition is considered important to the flavor and aroma profile.[7]
Safety
Like many esters, ethyl octanoate is not considered to be toxic. LD50 in rats is 25.96 g/kg. Though it has a low explosive limit of only 0.67 vol%, it is also weakly volatile, with a vapor pressure of only 0.2 mbar at room temperature. Ethyl octanoate is combustible.
References
- ↑ 1.0 1.1 Sheu, Yaw-Wen; Tu, Chein-Hsiun (2006). "Densities and Viscosities of Binary Mixtures of Isoamyl Acetate, Ethyl Caproate, Ethyl Benzoate, Isoamyl Butyrate, Ethyl Phenylacetate, and Ethyl Caprylate with Ethanol at T = (288.15, 298.15, 308.15, and 318.15) K". Journal of Chemical and Engineering Data 51 (2): 496–503. doi:10.1021/je050389b.
- ↑ 2.0 2.1 Ethyl octanoate from PubChem, accessed 30 December 2023
- ↑ Mark, James E. (2007). Physical Properties of Polymer Handbook (2nd ed.). Springer. p. 294. ISBN 978-0387690025. https://books.google.com/books?id=fZl7q7UgEXkC&q=hildebrand+parameters+of+representative+polymers&pg=PA289. Retrieved 1 March 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Record of Ethyl octanoate in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 10 August 2010.
- ↑ Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (15 January 2003). "Flavors and Fragrances". Flavors and Flagrances. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. doi:10.1002/14356007.a11_141. ISBN 3527306730. http://www.mrw.interscience.wiley.com/emrw/9783527306732/ueic/article/a11_141/current/abstract.
- ↑ "Ethyl octanoate - Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.003.078.
- ↑ Marais, J. et al. (2003) http://www.sawislibrary.co.za/dbtextimages/MaraisJ10.pdf "Effect of Different Wine-Making Techniques on the Composition and Quality of Pinotage Wine. I. Low-Temperature Skin Contact Prior to Fermentation"
 |

