Chemistry:Sec-Amyl acetate
From HandWiki
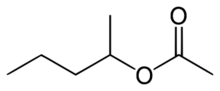
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentan-2-yl acetate | |
| Other names
1-Methylbutyl acetate
2-Pentanol acetate 2-Pentyl ester of acetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H14O2 | |
| Molar mass | 130.187 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Odor | Mild,[1] like bananas[2] |
| Density | 0.87 g/mL (20°C)[1] |
| Melting point | −78 °C; −109 °F; 195 K[1] |
| Boiling point | 121 °C; 249 °F; 394 K[1] |
| 0.2g/100g water (20°C)[2] | |
| Vapor pressure | 7 mmHg (20°C)[1] |
| Hazards | |
| GHS Signal word | Warning[2] |
| H226[2] | |
| Flash point | 32 °C; 89 °F; 305 K[1] |
| 380 °C (716 °F; 653 K) | |
| Explosive limits | 1–7.5% (20°C)[1] |
| Lethal dose or concentration (LD, LC): | |
LCLo (lowest published)
|
9200 ppm (guinea pig, 7 hr) 10,000 ppm (guinea pig, 5 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 125 ppm (650 mg/m3)[1] |
REL (Recommended)
|
TWA 125 ppm (650 mg/m3)[1] |
IDLH (Immediate danger)
|
1000 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
sec-Amyl acetate is an organic compound and an ester. It is formed in an esterification reaction of sec-amyl alcohol (2-pentanol) and acetic acid.[2] It is a colorless liquid.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 NIOSH Pocket Guide to Chemical Hazards. "#0032". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0032.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 "2-Pentyl Acetate". PubChem. NCBI. https://pubchem.ncbi.nlm.nih.gov/compound/2-pentyl_acetate.
- ↑ "sec-Amyl acetate". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/626380.html.
 |
