Chemistry:Propyl acetate
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Propyl acetate | |
| Systematic IUPAC name
Propyl ethanoate | |
| Other names
Acetic acid propyl ester
n-Propyl ethanoate n-Propyl acetate n-Propyl ester of acetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1276 |
| |
| |
| Properties | |
| C5H10O2 | |
| Molar mass | 102.133 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Mild, fruity[1] |
| Density | 0.89 g/cm3[2] |
| Melting point | −95 °C (−139 °F; 178 K)[2] |
| Boiling point | 102 °C (216 °F; 375 K)[2] |
| 18.9 g/L[2] | |
| Vapor pressure | 25 mmHg (20 °C)[1] |
| −65.91·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H319, H336 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+361+353, P304+340, P305+351+338, P312, P337+313, P370+378, P403+233, P403+235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 10 °C (50 °F; 283 K)[2] |
| 450 °C (842 °F; 723 K) | |
| Explosive limits | 1.7–8%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
9370 mg/kg (oral, rat) 8300 mg/kg (oral, mouse) 6640 mg/kg (oral, rabbit) 8700 mg/kg (oral, rat)[3] 17800 mg/kg (dermal, rabbit)[4] |
LCLo (lowest published)
|
8941 ppm (cat, 5 hr)[5] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 200 ppm (840 mg/m3)[1] |
REL (Recommended)
|
TWA 200 ppm (840 mg/m3) ST 250 ppm (1050 mg/m3)[1] |
IDLH (Immediate danger)
|
1700 ppm[1] |
| Related compounds | |
Related esters
|
Ethyl acetate Isopropyl acetate n-butyl acetate Isobutyl acetate |
Related compounds
|
Propan-1-ol Acetic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
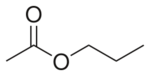
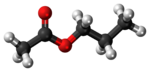
Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and propan-1-ol, often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct.[6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 NIOSH Pocket Guide to Chemical Hazards. "#0532". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0532.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ "n-Propyl acetate". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/109604.html.
- ↑ Union Carbide Data Sheet. Vol. 1/25/1965
- ↑ "n-Propyl acetate". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/109604.html.
- ↑ Papa, Anthony J. (2011-10-15), Propanols, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, doi:10.1002/14356007.a22_173.pub2, ISBN 978-3-527-30385-4, https://doi.org/10.1002/14356007.a22_173.pub2, retrieved 2022-03-29
External links
- NIOSH Pocket Guide to Chemical Hazards
- Acetic acid, propyl ester - Toxicity Data
- N-Propyl Acetate MSDS
 |

