Chemistry:Isoamyl acetate
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylbutyl acetate | |
| Systematic IUPAC name
3-Methylbutyl ethanoate | |
| Other names
Isopentyl acetate
Isopentyl ethanoate Isoamyl acetate Banana oil Pear essence | |
| Identifiers | |
3D model (JSmol)
|
|
| 1744750 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 101452 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1104 1993 |
| |
| |
| Properties | |
| C7H14O2 | |
| Molar mass | 130.187 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Banana-like[1] |
| Density | 0.876 g/cm3 |
| Melting point | −78 °C (−108 °F; 195 K) |
| Boiling point | 142 °C (288 °F; 415 K) |
| 0.3% (20 °C)[1] | |
| Vapor pressure | 4 mmHg or 0.533 kPa (20 °C)[1] |
| −89.4·10−6 cm3/mol | |
Refractive index (nD)
|
1.4020 at 20° |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H226, H315, H319, H335, H336, H372 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P314, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235 | |
| NFPA 704 (fire diamond) | |
| Flash point | 25 °C (77 °F; 298 K) |
| Explosive limits | 1.0% (100 °C) – 7.5%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
7422 mg/kg (rabbit, oral) 16,600 mg/kg (rat, oral)[2] |
LCLo (lowest published)
|
6470 ppm (cat)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (525 mg/m3)[1] |
REL (Recommended)
|
TWA 100 ppm (525 mg/m3)[1] |
IDLH (Immediate danger)
|
1000 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
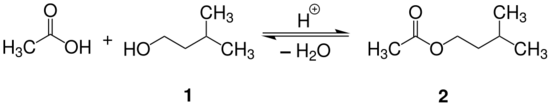
Isoamyl acetate, also known as isopentyl acetate, is an organic compound that is the ester formed from isoamyl alcohol and acetic acid, with the molecular formula [math]\ce{ C7H14O2 }[/math]. It is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as similar to both banana and pear.[3] Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl acetate, and other flavors in ethanol may be referred to as banana oil[4] or pear oil.[5]
Production
Isoamyl acetate is prepared by the acid catalyzed reaction (Fischer esterification) between isoamyl alcohol and glacial acetic acid as shown in the reaction equation below. Typically, sulfuric acid is used as the catalyst. Alternatively, p-toluenesulfonic acid or an acidic ion exchange resin can be used as the catalyst.
It is also produced synthetically by the rectification of amyl acetate.[6]
Applications
Isoamyl acetate is used to confer banana or pear flavor in foods such as circus peanuts, Juicy Fruit and pear drops.[7] Banana oil and pear oil commonly refer to a solution of isoamyl acetate in ethanol that is used as an artificial flavor.
It is also used as a solvent for some varnishes, oil paints, and nitrocellulose lacquers. As a solvent and carrier for materials such as nitrocellulose, it was extensively used in the aircraft industry for stiffening and wind-proofing fabric flying surfaces, where it and its derivatives were generally known as 'aircraft dope'. Now that most aircraft wings are made of metal, such use is mostly limited to historically accurate reproductions and scale models.
Because of its intense, pleasant odor and its low toxicity, isoamyl acetate is used to test the effectiveness of respirators or gas masks.[8]
Occurrence in nature
Isoamyl acetate occurs naturally in many plants, including apple, banana, coffee, grape, guava, lychee, papaya, peach, pomegranate, and tomato.[9][10] It is also released by fermentation processes, including those used for making beer, cognac, and whisky.[9]
Isoamyl acetate is released by a honey bee's sting apparatus where it serves as a pheromone beacon to attract other bees and provoke them to sting.[11]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 NIOSH Pocket Guide to Chemical Hazards. "#0347". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0347.html.
- ↑ 2.0 2.1 "Isoamyl acetate". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/123922.html.
- ↑ "Iso-amyl acetate". chemicalland21.com. http://www.chemicalland21.com/specialtychem/perchem/ISO-AMYL%20ACETATE.htm.
- ↑ Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_141.
- ↑ "T3DB: Isopentyl acetate". Canadian Institutes of Health Research, Canada Foundation for Innovation, and by The Metabolomics Innovation Centre (TMIC). http://www.t3db.ca/toxins/T3D4851.
- ↑ "Isoamyl acetate Methods of Manufacturing" (in en). National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/compound/Isoamyl-acetate#section=Methods-of-Manufacturing.
- ↑ "Isoamyl acetate" (in en). https://www.acs.org/content/acs/en/molecule-of-the-week/archive/i/isoamyl-acetate.html.
- ↑ "Fit Testing Procedures (Mandatory). - 1910.134 App A | Occupational Safety and Health Administration". https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=9780&p_table=STANDARDS.
- ↑ 9.0 9.1 "SUMMARY OF DATA FOR CHEMICAL SELECTION: Isoamyl Acetate". U.S. Department of Health and Human Services. November 1994. https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/isoamylacetate_508.pdf.
- ↑ "Isoamyl acetate Taxonomy" (in en). National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/compound/Isoamyl-acetate#section=Taxonomy&fullscreen=true.
- ↑ Boch R; Shearer DA; Stone BC (September 8, 1962). "Identification of isoamyl acetate as an active component in the sting pheromone of the honey bee". Nature (England: Nature Publishing Group) 195 (4845): 1018–20. doi:10.1038/1951018b0. PMID 13870346. Bibcode: 1962Natur.195.1018B.
 |