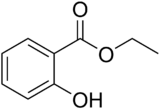
Chemistry:Ethyl salicylate
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl 2-hydroxybenzoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10O3 | |
| Molar mass | 166.176 g·mol−1 |
| Appearance | Colorless liquid[2] |
| Odor | wintergreen[3] |
| Density | 1.13 g/cm3[2] |
| Melting point | 1.3 °C (34.3 °F; 274.4 K)[2] |
| Boiling point | 232 °C (450 °F; 505 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Ethyl salicylate is the ester formed by the condensation of salicylic acid and ethanol. It is a clear liquid that is sparingly soluble in water, but soluble in alcohol and ether. It has a pleasant odor resembling wintergreen and is used in perfumery and artificial flavors.[3]
See also
References
- ↑ Pubchem. "Ethyl salicylate". https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl_salicylate. Retrieved 25 February 2018.
- ↑ 2.0 2.1 2.2 2.3 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ 3.0 3.1 Lapczynski, A; McGinty, D; Jones, L; Bhatia, S; Letizia, C.S; Api, A.M (2007). "Fragrance material review on ethyl salicylate". Food and Chemical Toxicology 45: S397–401. doi:10.1016/j.fct.2007.09.043. PMID 18023517.
 |

