Chemistry:Salsalate
 | |
| Clinical data | |
|---|---|
| Trade names | Disalcid, Salflex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682880 |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
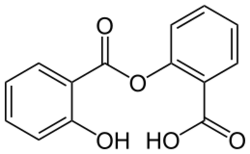
| Formula | C14H10O5 |
| Molar mass | 258.229 g·mol−1 |
| | |
Salsalate is a medication that belongs to the salicylate and nonsteroidal anti-inflammatory drug (NSAID) classes.
Salsalate is the generic name of a prescription drug marketed under the brandnames Mono-Gesic, Salflex, Disalcid, and Salsitab. Other generic and brand name formulations may be available.[1]
Mechanism of action
Relative to other NSAIDs, salsalate has a weak inhibitory effect on the cyclooxygenase enzyme and decreases the production of several proinflammatory chemical signals such as interleukin-6, TNF-alpha, and C-reactive protein.[2]
The mechanism through which salsalate is thought to reduce the production of these inflammatory chemical signals is through the inhibition of IκB kinase resulting in decreased action of NF-κB genes.[2][3][4] This mechanism is thought to be responsible for salsalate's insulin-sensitizing and blood sugar lowering properties.[3]
Medical uses
Salsalate may be used for inflammatory disorders such as rheumatoid arthritis or noninflammatory disorders such as osteoarthritis.[2][5]
Safety
The risk of bleeding is a common concern with use of the NSAID class of medications. However, the bleeding risk associated with salsalate is lower than that associated with aspirin use.[3]
Research
Salsalate has been proposed for the prevention and treatment of type 2 diabetes mellitus due to its ability to lower insulin resistance associated with inflammation and may be useful in prediabetes.[2] However, the use of salsalate to prevent the progression from prediabetes to type 2 diabetes mellitus has received limited study.[2]
History
This section is missing information about first known synthesis and commercialization — who decided to stick two salicylic acid molecules together?. (January 2023) |
Salsalate had been suggested as possible treatment for diabetes as early as 1876.[2][6][7]
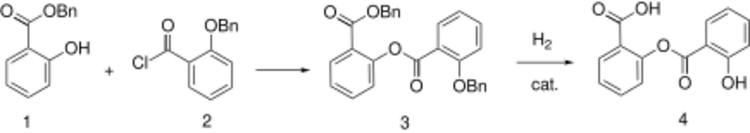
Synthesis
References
- ↑ "Salsalate". drugs.com. https://www.drugs.com/mtm/salsalate.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Salsalate, an old, inexpensive drug with potential new indications: a review of the evidence from 3 recent studies". American Health & Drug Benefits 7 (4): 231–5. June 2014. PMID 25126374.
- ↑ 3.0 3.1 3.2 "Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease". Expert Opinion on Investigational Drugs 24 (3): 283–307. March 2015. doi:10.1517/13543784.2015.974804. PMID 25345753.
- ↑ "Anti-inflammatory therapies for cardiovascular disease". European Heart Journal 35 (27): 1782–91. July 2014. doi:10.1093/eurheartj/ehu203. PMID 24864079.
- ↑ "AMPK: a target for drugs and natural products with effects on both diabetes and cancer". Diabetes 62 (7): 2164–72. July 2013. doi:10.2337/db13-0368. PMID 23801715.
- ↑ "Obesity: the two faces of fat". Nature 447 (7144): 525–7. May 2007. doi:10.1038/447525a. PMID 17538594. Bibcode: 2007Natur.447..525P.
- ↑ "Zur therapie des diabetes mellitus, insbesondere uber die anwendung des salicylsauren natron bei demselben". Berliner Klinische Wochenschrift 13: 337–340. 1876.
- ↑ "Synthesis of Phenolic Acid Esters. I. Depsides1". Journal of the American Chemical Society 65 (11): 2140–2142. 1943. doi:10.1021/ja01251a034.
- ↑ "42. Eight- and higher-membered ring compounds. Part II. Di-, tri-, tetra-, and hexa-salicylides". Journal of the Chemical Society (Resumed): 201. 1951. doi:10.1039/JR9510000201.
- ↑ "Verfarhen zur Darstellung einer kristallisierten Salicylosalicylsäure aus Salicylsäure oder ihrne Salzen [Process for preparing a crystallized salicylosalicylic acid from salicylic acid or its salts]" DE patent 211403, published 1909-06-25
- ↑ "Verfarhen zur Darstellung einer kristallisierten Salicylosalicylsäure [Process for preparing a crystallized salicylosalicylic acid]" DE patent 214044, published 1909-09-20
 |
