Chemistry:Fertirelin
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Ovalyse |
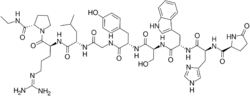
| Other names | TAP-031; U-69689; 9-(N-Ethyl-L-prolinamide)-10-deglycinamide; 9-(N)-Et-ProNH2-10-des-GlyNH2-LHRH; Pyr-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-NHEt |
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| ATCvet code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C55H76N16O12 |
| Molar mass | 1153.313 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fertirelin, or fertirelin acetate, sold under the brand name Ovalyse, is a gonadotropin-releasing hormone agonist (GnRH agonist) which has been marketed in the United Kingdom and Austria.[1][2][3] It may no longer be available.[4] Fertirelin has been used in veterinary medicine.[2] It may have been used in the treatment of sex hormone-dependent conditions and infertility in women.[3] The drug was first introduced in 1981 in Japan to treat various kinds of ovarian failure in cattle.[5] Fertirelin is a synthetic peptide and GnRH analogue.[1][2] It is used as the acetate salt.[2]
See also
References
- ↑ 1.0 1.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 549–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA549.
- ↑ 2.0 2.1 2.2 2.3 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 441–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA441.
- ↑ 3.0 3.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 120–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA120.
- ↑ "List of Gonadotropin releasing hormones". Drugs.com. https://www.drugs.com/international/fertirelin.html.
- ↑ "New degradation product of des-Gly10-NH2-LH-RH-ethylamide (fertirelin) in aqueous solution". Journal of Pharmaceutical Sciences 80 (2): 167–170. February 1991. doi:10.1002/jps.2600800217. PMID 2051323.
 |
