Chemistry:Nafarelin
 | |
| Clinical data | |
|---|---|
| Trade names | Synarel, Nasanyl, others |
| Other names | Nafareline; Nafarelin acetate; RS-94991; RS-94991-298; [6-D-(2-naphthyl)alanine]-GnRH |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601082 |
| Pregnancy category |
|
| Routes of administration | Nasal spray[1][2] |
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IN: 2.8% (1.2–5.6%)[2] |
| Protein binding | 80%[2] |
| Metabolism | Peptidases (not CYP450)[2] |
| Elimination half-life | IN: 2.5–3.0 hours[2] SC: 86 hours (metabolites)[2] |
| Excretion | Urine: 44–55%[2] Feces: 19–44%[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C66H83N17O13 |
| Molar mass | 1322.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Nafarelin, sold under the brand name Synarel among others, is a gonadotropin-releasing hormone agonist (GnRH agonist) medication which is used in the treatment of endometriosis and early puberty.[1][2] It is also used to treat uterine fibroids, to control ovarian stimulation in in vitro fertilization (IVF), and as part of transgender hormone therapy.[3][4][5][6] The medication is used as a nasal spray two to three times per day.[1][2][7]
Side effects of nafarelin are related to sex hormone deprivation and include symptoms of low testosterone levels and low estrogen levels such as hot flashes, sexual dysfunction, vaginal atrophy, and osteoporosis.[2] Nafarelin is a gonadotropin-releasing hormone agonist (GnRH agonist) and works by preventing the production of sex hormones by the gonads.[1][2] It can lower sex hormone levels by 95% in both sexes.[1][2] Nafarelin is a peptide and an analogue of GnRH.[8]
Nafarelin was introduced for medical use in 1990.[9][1][10] It is available widely throughout the world, including in North America, Europe, and elsewhere throughout the world.[11][12] The medication is one of only two medically used GnRH analogues that are available as nasal sprays, the other being buserelin.[13]
Medical uses
Nafarelin is approved and used in the treatment of endometriosis and precocious puberty.[2][1] It is also used in the treatment of uterine fibroids.[3][14] The medication is used to control ovarian stimulation in in vitro fertilization (IVF).[4][15] Nafarelin is used as a puberty blocker in transgender youth and to suppress testosterone levels in transgender women.[16][5][17][6][18] Nafarelin can also be used to treat hirsutism and polycystic ovary syndrome by lowering gonadotropin and androgen levels.[1][14][19] It is effective in the treatment of benign prostatic hyperplasia.[1]
Dosages
Nafarelin is used to treat precocious puberty at a dosage of 1,600 to 1,800 μg per day.[2] The 1,600 μg/day dosage is achieved by two sprays (400 μg total) into each nostril in the morning (four sprays, 800 μg total) and two sprays (400 μg total) into each nostril in the evening (four sprays, 800 μg total).[2] If 1,600 μg/day is insufficient for adequate pubertal suppression, the 1,800 μg/day dosage can be used instead. This is achieved by three sprays (600 μg total) into alternating nostrils three times per day (nine sprays per day total).[2] When administering the sprays, the head should be tilted back slightly and 30 seconds should elapse between each spray.[2] A bottle of nafarelin nasal spray (brand name Synarel) lasts for about 7 days at a dosage of 1,600 μg/day.[2]
Nafarelin is used to treat endometriosis at lower dosages of 400 to 800 μg per day.[2] This is achieved by one or two sprays (200 or 400 μg total) into alternating nostrils once in the morning and once in the evening (two to four sprays per day total).[2] A bottle of nafarelin nasal spray (brand name Synarel) lasts for about 30 days at a dosage of 400 μg/day.[2]
Available forms
Nafarelin is available in the form of a 0.2% nasal spray for use one, two, or three times per day.[20][2][1] Each bottle of nafarelin nasal spray (brand name Synarel) contains about 60 sprays delivering approximately 200 μg nafarelin in 100 μL solution per actuation.[2] Nafarelin is not available for use by any other routes than intranasal administration.[21]
Side effects
Side effects of nafarelin are related to sex hormone deficiency and include hot flashes, vaginal dryness, headaches, mood changes, and sexual dysfunction. Nafarelin causes erectile dysfunction in more than half of men with benign prostatic hyperplasia treated with it.[22] Some people may experience acne, muscle pain, reduced breast size, and nasal irritation. These side effects are reversible and should resolve after stopping the medication.[23] There is a case report of severe hyperkalemia during nafarelin therapy in a woman with uterine fibroids.[24] The mechanism is unknown.[24]
Pharmacology
Pharmacodynamics
Nafarelin is a GnRH agonist, or an agonist of the GnRH receptor, the biological target of GnRH.[1][2] It works by continuously activating the GnRH receptor, which results in profound desensitization of the receptor such that it becomes non-functional.[1][2] As a result, nafarelin suppresses the GnRH-induced secretion of the gonadotropins, luteinizing hormone and follicle-stimulating hormone, from the pituitary gland.[1][2] This, in turn, results in profound suppression of gonadal sex hormone production, as well as reversible suppression of fertility.[1][2]
Pharmacokinetics
The bioavailability of nafarelin with intranasal administration is 2.8% on average, with a range of 1.2 to 5.6%.[2] The plasma protein binding of nafarelin is 80%.[2] It is metabolized primarily by peptidases and not by cytochrome P450 enzymes.[2] The elimination half-life of nafarelin is 2.5 to 3.0 hours by intranasal administration, whereas the half-life of nafarelin and its metabolites by subcutaneous injection is 85.5 hours.[2] Nafarelin is eliminated 44 to 55% in urine and 18.5 to 44.2% in feces.[2]
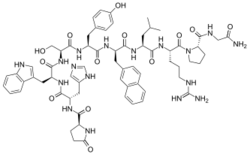
Chemistry
Nafarelin is a GnRH analogue, or a synthetic analogue of GnRH.[1][2][21] It is a decapeptide and is also known as [6-D-(2-naphthyl)alanine]-GnRH.[21][25] Nafarelin is marketed for medical use in both its free base (nafarelin) and acetate salt (nafarelin acetate) forms.[12]
History
Nafarelin was introduced for medical use in 1990.[9][1][10]
Society and culture
Generic names
Nafarelin is the generic name of the drug and its INN and BAN, while nafaréline is its DCF and nafarelin acetate is its USAN, BANM, and JAN.[26][12][27][11] It is also known by its former developmental code name RS-94991 or RS-94991-298.[26][12][27][11]
Brand names
The major brand names of nafarelin are Synarel and Synarela.[12][11] It has also been marketed under a number of other brand names including Synrelin, Synrelina, Nafarelil 0.2%, and Nasanyl 0.2%.[12][11]
Availability
Nafarelin is available widely throughout the world, including in the United States , Canada , the United Kingdom , Ireland, other European countries, Australia , Israel, Argentina , Brazil , Mexico, and Japan .[12][11]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 "Nafarelin. A review of its pharmacodynamic and pharmacokinetic properties, and clinical potential in sex hormone-related conditions". Drugs 39 (4): 523–551. April 1990. doi:10.2165/00003495-199039040-00005. PMID 2140979.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 2.28 2.29 2.30 2.31 2.32 2.33 https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019886s033s035lbl.pdf [bare URL PDF]
- ↑ 3.0 3.1 "Clinical use of nafarelin in the treatment of leiomyomas. A review of the literature". The Journal of Reproductive Medicine 45 (6): 481–489. June 2000. PMID 10900582.
- ↑ 4.0 4.1 (in de) Arzneimittelwirkungen (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. 2001. pp. 372–3. ISBN 3-8047-1763-2.
- ↑ 5.0 5.1 "Gender Incongruity in Children With and Without Disorders of Sexual Differentiation". Endocrinology and Metabolism Clinics of North America 45 (2): 463–482. June 2016. doi:10.1016/j.ecl.2016.02.001. PMID 27241976.
- ↑ 6.0 6.1 "Management of transgenderism". JAMA 309 (5): 478–484. February 2013. doi:10.1001/jama.2012.165234. PMID 23385274.
- ↑ "Gonadotropin releasing hormone agonists: Expanding vistas". Indian Journal of Endocrinology and Metabolism 15 (4): 261–267. October 2011. doi:10.4103/2230-8210.85575. PMID 22028996.
- ↑ Peptide-based Drug Discovery: Challenges and New Therapeutics. Royal Society of Chemistry. 26 June 2017. pp. 182–. ISBN 978-1-78262-732-6. https://books.google.com/books?id=sbcpDwAAQBAJ&pg=PA182.
- ↑ 9.0 9.1 "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=019886.
- ↑ 10.0 10.1 "Gonadotropin-releasing hormone and its analogues". The New England Journal of Medicine 324 (2): 93–103. January 1991. doi:10.1056/NEJM199101103240205. PMID 1984190.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Nafarelin nasal Uses, Side Effects & Warnings". https://www.drugs.com/international/nafarelin.html.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 712–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA712.
- ↑ Nasal Physiology and Pathophysiology of Nasal Disorders. Springer Science & Business Media. 17 August 2013. pp. 208–. ISBN 978-3-642-37250-6. https://books.google.com/books?id=qfu8BAAAQBAJ&pg=PA208.
- ↑ 14.0 14.1 "Treatment of uterine leiomyomas and hirsutism with nafarelin". The Journal of Reproductive Medicine 34 (12 Suppl): 1029–1033. December 1989. PMID 2533618.
- ↑ "The neurochemistry of the GnRH pulse generator". Acta Neurobiologiae Experimentalis 56 (3): 707–713. 1996. PMID 8917899. http://www.ane.pl/linkout.php?vol=56&no=3&fpp=707.
- ↑ "Care of Gender Nonconforming/Transgender Youth". Pediatric Endocrinology. 2018. pp. 813–823. doi:10.1007/978-3-319-73782-9_36. ISBN 978-3-319-73781-2.
- ↑ "The peripubertal gender-dysphoric child: puberty suppression and treatment paradigms". Pediatric Annals 43 (6): e132–e137. June 2014. doi:10.3928/00904481-20140522-08. PMID 24972421.
- ↑ "Endocrine treatment of transsexuals: assessment of cardiovascular risk factors". Expert Review of Endocrinology & Metabolism 5 (3): 319–322. May 2010. doi:10.1586/eem.10.18. PMID 30861686.
- ↑ "GnRH agonists-antagonists--clinical applications". European Journal of Obstetrics, Gynecology, and Reproductive Biology 28 (2): 109–116. June 1988. doi:10.1016/0028-2243(88)90086-X. PMID 2969835.
- ↑ Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. 24 January 2012. pp. 230–. ISBN 978-1-60913-345-0. https://books.google.com/books?id=Sd6ot9ul-bUC&pg=PA230.
- ↑ 21.0 21.1 21.2 Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer. 14 June 2017. pp. 9–. ISBN 978-3-319-52210-4. https://books.google.com/books?id=pzgoDwAAQBAJ&pg=PA9.
- ↑ "Impact of Androgen Deprivation on Male Sexual Function". Sexual Function in the Prostate Cancer Patient. 2009. pp. 163–175. doi:10.1007/978-1-60327-555-2_11. ISBN 978-1-60327-554-5.
- ↑ DailyMed: Synarel – nafarelin acetate spray
- ↑ 24.0 24.1 "Severe hyperkalaemia with nafarelin". Lancet 347 (8997): 333. February 1996. doi:10.1016/S0140-6736(96)90514-0. PMID 8569395.
- ↑ "The role of LHRH-analogues in protecting gonadal functions during chemotherapy and irradiation". European Urology 23 (1): 157–63; discussion 163–4. 1993. doi:10.1159/000474586. PMID 8477775.
- ↑ 26.0 26.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 846–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA846.
- ↑ 27.0 27.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 189–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA189.
Further reading
- "Comparison of the pharmacology of nafarelin and danazol". American Journal of Obstetrics and Gynecology 162 (2): 581–585. February 1990. doi:10.1016/0002-9378(90)90436-B. PMID 2137975.
- "Nafarelin. A review of its pharmacodynamic and pharmacokinetic properties, and clinical potential in sex hormone-related conditions". Drugs 39 (4): 523–551. April 1990. doi:10.2165/00003495-199039040-00005. PMID 2140979.
- "Nafarelin in the management of endometriosis: quality of life assessment". American Journal of Obstetrics and Gynecology 166 (2): 735–739. February 1992. doi:10.1016/0002-9378(92)91705-F. PMID 1531576.
- "Clinical use of nafarelin in the treatment of leiomyomas. A review of the literature". The Journal of Reproductive Medicine 45 (6): 481–489. June 2000. PMID 10900582.
