Chemistry:Triptorelin
 | |
| Clinical data | |
|---|---|
| Trade names | Decapeptyl, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| Routes of administration | Intramuscular |
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C64H82N18O13 |
| Molar mass | 1311.473 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Triptorelin, sold under the brand name Decapeptyl among others, is a medication that acts as an agonist analog of gonadotropin-releasing hormone, repressing expression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).[3][4]
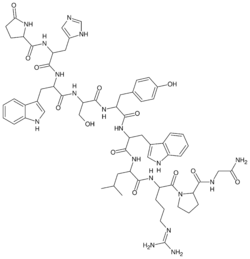
It is a decapeptide (pGlu-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2) and a gonadotropin-releasing hormone agonist (GnRH agonist) used as the acetate or pamoate salts.
Primary indications include endometriosis,[5] for the reduction of uterine fibroids, to treat prostate cancer, and to treat male hypersexuality with severe sexual deviation.[4] The drug has also been used off label to delay puberty in patients with gender dysphoria.[6]
It was patented in 1975 and approved for medical use in 1986.[7] It is on the World Health Organization's List of Essential Medicines.[8]
Medical uses
Triptorelin is used to treat prostate cancer as part of androgen deprivation therapy.[9]
Another common use in the United Kingdom is for hormone replacement therapy to suppress testosterone or estrogen levels in transgender people (in conjunction with estradiol valerate for trans women or testosterone for trans men). Spironolactone and cyproterone acetate are other drugs used by trans people to suppress sex hormones, but these drugs have a completely different mechanism of action.[10] It can also be used as a puberty blocker.[11]
Triptorelin has been used as a chemical castration agent for reducing sexual urges in sex offenders.[12]
Drug action
Triptorelin is a gonadorelin analogue, also known as luteinizing hormone releasing analogue (GnRH analogue, LHRH analogue).[3] The drug binds to receptors in the pituitary gland and stimulates secretion of gonadotropins (namely luteinizing hormone LH and follicle-stimulating hormone FSH). This causes an initial phase of LH and FSH stimulation, prior to down-regulation of the gonadotrophin-releasing hormone receptors, thereby reducing the release of gonadotropins in the long term, which in turn leads to the inhibition of androgen and estrogen production.[4]
Side-effects
General side effects can include:[4]
- Anaphylaxis
- Arthralgia
- Asthenia
- Asthma
- Breast tenderness (males and females)
- Changes in blood pressure
- Changes in breast size
- Depression
- Ovarian cysts
- Mood changes
- Skin rashes
- Hot flushes
- Weight changes
Society and culture
Brand names
Triptorelin is marketed under the brand names Decapeptyl (Ipsen) for treating prostate cancer, endometriosis, uterine myomas, and precocious puberty,[13] and Diphereline and Gonapeptyl (Ferring Pharmaceuticals).[14] In the United States, it is sold by Watson Pharmaceuticals as Trelstar [15] and by Arbor Pharmaceuticals as Triptodur (an extended-release 6-month depot injection).[16] In Iran, triptorelin is marketed under the brand name Variopeptyl. In the UK and Germany, it is sold as Salvacyl for the treatment of sexual deviations.[17]
History
Triptorelin was developed in Andrew V. Schally's lab at Tulane University.[18] Debiopharm licensed the drug from Tulane in 1982.[19]
Research
Triptorelin and other antiandrogens may be effective in the treatment of obsessive–compulsive disorder.[20]
References
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2015". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2015.
- ↑ "Triptodur- triptorelin kit". DailyMed. U.S. National Library of Medicine. 28 April 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f41380e7-b830-432d-a5f5-a872932f107e.
- ↑ 3.0 3.1 "gonadorelin analogue". https://www.encyclopedia.com/caregiving/dictionaries-thesauruses-pictures-and-press-releases/gonadorelin-analogue.
- ↑ 4.0 4.1 4.2 4.3 Joint Formulary Committee (2018). British National Formulary (BNF) 70. London: Pharmaceutical Press. pp. 635. ISBN 978-0-85711-173-9.
- ↑ "Triptorelin for the treatment of endometriosis". Expert Opinion on Pharmacotherapy (Informa Healthcare) 15 (8): 1153–1179. June 2014. doi:10.1517/14656566.2014.916279. PMID 24832495.
- ↑ "Gender dysphoria in children: puberty blockers study draws further criticism". BMJ 366: l5647. September 2019. doi:10.1136/bmj.l5647. PMID 31540909.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 514. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA514.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "triptorelin (Intramuscular route)". https://www.drugs.com/cons/triptorelin-intramuscular-injection.html.
- ↑ "Recommendations of endocrine treatment for patients with gender dysphoria". Sexual and Relationship Therapy 24 (2): 175–187. 2009. doi:10.1080/14681990903023306.
- ↑ "Advances in the Care of Transgender Children and Adolescents". Advances in Pediatrics 63 (1): 79–102. August 2016. doi:10.1016/j.yapd.2016.04.018. PMID 27426896.
- ↑ "Study: Drug effectively treats pedophilia". CNN. 11 February 1998. http://edition.cnn.com/HEALTH/9802/11/sex.offender.drug/.
- ↑ "Decapeptyl SR 11.25mg (triptorelin pamoate) - Patient Information Leaflet (PIL) - (emc)". https://www.medicines.org.uk/emc/product/780/pil#gref.
- ↑ "Gonapeptyl Depot 3.75 mg - Summary of Product Characteristics (SmPC) - (emc)". https://www.medicines.org.uk/emc/product/2229/smpc#gref.
- ↑ "Trelstar intramuscular: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing" (in en). WebMD. https://www.webmd.com/drugs/2/drug-153984-8162/trelstar-intramuscular/triptorelin-3-month-11-25-mg-injection/details.
- ↑ "Triptodur Intramuscular: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing" (in en). WebMD. https://www.webmd.com/drugs/2/drug-174078/triptodur-intramuscular/details.
- ↑ "Salvacyl®, developed by Debiopharm, is launched in two European countries - a new therapeutic avenue for the treatment of sexual deviations" (in en-US). 23 June 2009. https://www.debiopharm.com/drug-development/press-releases/salvacyl-developed-by-debiopharm-is-launched-in-two-european-countries-a-new-therapeutic-avenue-for-the-treatment-of-sexual-deviations/.
- ↑ "About Us – Technology Transfer at Tulane University". Tulane University. https://research.tulane.edu/oipm/about-us. Retrieved 4 January 2024.
- ↑ "Dr. Reddy's Launches Debiopharm's Pamorelin LA in India for the Treatment of Locally Advanced or Metastatic, Hormone-Dependent Prostate Cancer". Fierce Pharma. 7 December 2012. https://www.fiercepharma.com/pharma/dr-reddy-s-launches-debiopharm-s-pamorelin%C2%AE-la-india-for-treatment-of-locally-advanced-or. Retrieved 4 January 2024.
- ↑ "Anti-Androgen Drugs in the Treatment of Obsessive-Compulsive Disorder: A Systematic Review". Current Medicinal Chemistry 27 (40): 6825–6836. December 2019. doi:10.2174/0929867326666191209142209. PMID 31814547.
Further reading
- "Pharmacokinetics and pharmacodynamics of GnRH agonists: clinical implications in pediatrics". Journal of Pediatric Endocrinology & Metabolism 13 (Suppl 1): 723–737. July 2000. doi:10.1515/jpem.2000.13.s1.723. PMID 10969915.
- "GnRH analogues--agonists and antagonists". Animal Reproduction Science 88 (1–2): 115–126. August 2005. doi:10.1016/j.anireprosci.2005.05.005. PMID 15955640.
External links
- "Triptorelin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/triptorelin.
 |
