Chemistry:Bifluranol
 | |
| Clinical data | |
|---|---|
| Trade names | Prostarex |
| Other names | BX-341 |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
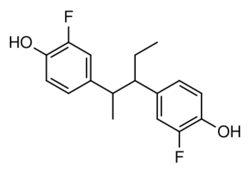
| Formula | C17H18F2O2 |
| Molar mass | 292.326 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bifluranol (INN, BAN; brand name Prostarex; former developmental code name BX-341) is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that has been used as an antiandrogen in the United Kingdom in the treatment of benign prostatic hyperplasia.[1][2][3][4][5][6][7][8] The drug is described as a weak estrogen, and possesses about one-eighth the potency of diethylstilbestrol.[3][7][9]
In spite of the fact that it is widely referred to as an antiandrogen in the literature, bifluranol is actually a pure estrogen and does not significantly bind to the androgen receptor or directly antagonize the action of androgens.[3] It exerts functional antiandrogen effects by binding to and activating the estrogen receptor in the pituitary gland, consequently suppressing the secretion of luteinizing hormone (and hence acting as an antigonadotropin) and thereby reducing gonadal androgen production and systemic androgen levels.[3] Bifluranol has also been found to act as a 17α-hydroxylase/17,20 lyase inhibitor, though with less potency than ketoconazole, and this action may contribute to its efficacy in benign prostatic hyperplasia by further helping to lower androgen levels.[10][11][12]
Related drugs include pentafluranol (BX-430) and terfluranol (BX-428), which are also estrogens.[13]
See also
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 152. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=RA1-PA364.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 124–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA124.
- ↑ 3.0 3.1 3.2 3.3 "Anti-prostatic activity of bifluranol, a fluorinated bibenzyl". British Journal of Pharmacology 71 (1): 11–16. 1980. doi:10.1111/j.1476-5381.1980.tb10903.x. PMID 6258683.
- ↑ "Bifluranol, a novel fluorinated bibenzyl anti-androgen, its chemistry and disposition in different animal species". The Journal of Pharmacy and Pharmacology 33 (5): 297–301. May 1981. doi:10.1111/j.2042-7158.1981.tb13784.x. PMID 6116777.
- ↑ "Bifluranol in the treatment of benign prostatic hyperplasia (BPH)". The Prostate 7 (4): 357–361. 1985. doi:10.1002/pros.2990070403. ISSN 0270-4137.
- ↑ "Response of the benign hypertrophied prostate to treatment with an LHRH analogue". British Journal of Urology 62 (2): 163–165. August 1988. doi:10.1111/j.1464-410X.1988.tb04299.x. PMID 2457404.
- ↑ 7.0 7.1 Spain. Ministerio de Agricultura; Universidad Complutense de Madrid. Departamento de Genetico y Mejora (1978). 3rd World Congress of Animal Feeding. Industrias Gráficas España. p. 532. ISBN 978-84-7391-022-4. https://books.google.com/books?id=ZgYFAQAAIAAJ.
- ↑ Annual Reports in Medicinal Chemistry. Academic Press. 16 September 1986. pp. 182–. ISBN 978-0-08-058365-5. https://books.google.com/books?id=qsFCGskRHZQC&pg=PA182.
- ↑ Receptor mediated antisteroid action. De Gruyter. 1987. p. 330. ISBN 978-0-89925-374-9. https://books.google.com/books?id=JfNsAAAAMAAJ.
- ↑ "Inhibition of 17 alpha-hydroxylase/C17-C20 lyase by bifluranol and its analogues". Journal of Steroid Biochemistry 33 (6): 1191–1195. December 1989. doi:10.1016/0022-4731(89)90429-9. PMID 2559252.
- ↑ "Inhibitors of enzymes of androgen biosynthesis: cytochrome P450(17) alpha and 5 alpha-steroid reductase". Natural Product Reports 15 (5): 495–512. October 1998. doi:10.1039/a815495y. PMID 9807812.
- ↑ "Biochemistry and pharmacokinetics of potent non-steroidal cytochrome P450(17alpha) inhibitors". The Journal of Steroid Biochemistry and Molecular Biology 60 (5–6): 347–351. March 1997. doi:10.1016/S0960-0760(96)00225-7. PMID 9219927.
- ↑ Polska Akademia Nauk. Komitet Badania Polonii (1984). II Kongres Uczonych Polskiego Pochodzenia: zbiór materiałów. Zakład Narodowy im. Ossolińskich. ISBN 978-83-04-01670-5. https://books.google.com/books?id=_-VjAAAAIAAJ. "[This explains why the estrogenic activity is minimal in terms of pentafluranol or even bifluranol. Doses which shall apply from 1 to 6 days of pregnancy, are in micrograms per kg of body weight: bifluranol 80, 30 and terfluranol pentafluranol 280 ...]"
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |

