Chemistry:Linzagolix
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌlɪnzəˈɡoʊlɪks/ LINZ-ə-GOH-liks |
| Trade names | Yselty |
| Other names | KLH-2109; OBE-2109 |
| Routes of administration | By mouth[1][2] |
| Drug class | GnRH modulator; GnRH antagonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL |
|
| Chemical and physical data | |
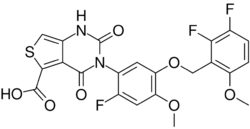
| Formula | C22H15F3N2O7S |
| Molar mass | 508.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Linzagolix, sold under the brand name Yselty, is a medication used in the treatment of uterine fibroids.[1][5] Linzagolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist (GnRH antagonist) developed by Kissei Pharmaceutical and ObsEva.[6][7][2]
In June 2022, it was approved for medical use in the European Union and in the United Kingdom.[1][4][8]
Medical uses
Linzagolix is indicated for treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age.[1]
Available forms
Linzagolix is available as linzagolix choline, the choline salt of linzagolix, in the form of 100 and 200 mg film-coated oral tablets.[5]
Pharmacology
Pharmacodynamics
Linzagolix acts as a selective antagonist of the GnRH receptor, the biological target of GnRH.[5] By blocking this receptor, linzagolix prevents GnRH-mediated secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and prevents them from signaling the gonads to produce sex hormones including estrogens, progesterone, and androgens.[5][9]
In clinical studies, linzagolix fully suppressed estradiol levels (median <20 pg/mL) in women at a dosage of 200 mg/day, whereas partial suppression of estradiol levels (median 20–60 pg/mL) occurred at a dosage 100 mg/day.[5] Progesterone levels were also variably suppressed with these dosages.[5]
Pharmacokinetics
The elimination half-life of linzagolix with repeated administration is approximately 15 hours.[5]
Society and culture
Legal status
On 16 December 2021, and on 22 April 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Yselty, intended for the treatment of symptoms of uterine fibroids.[10] The applicant for this medicinal product is ObsEva Ireland Ltd.[10] Linzagolix was approved for medical use in the European Union in June 2022.[1][4]
Brand names
Linzagolix is sold under the brand name Yselty.[5]
Availability
Linzagolix is available in the European Union and in the United Kingdom.[5][8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Yselty EPAR". 14 December 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/yselty. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 2.0 2.1 "Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain". Women's Health 11 (1): 19–28. January 2015. doi:10.2217/whe.14.68. PMID 25581052.
- ↑ "Linzagolix". 8 August 2022. https://www.sps.nhs.uk/medicines/linzagolix/.
- ↑ 4.0 4.1 4.2 "Yselty Product information". European Commission. https://ec.europa.eu/health/documents/community-register/html/h1606.htm.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "Yselty film-coated tablets". Theramex Ireland Limited. European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/yselty-epar-product-information_en.pdf.
- ↑ "Linzagolix - Kissei Pharmaceutical/ObsEva". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800032710.
- ↑ "New Horizons in Fibroid Management". Current Obstetrics and Gynecology Reports 7 (2): 106–115. 2018. doi:10.1007/s13669-018-0242-6. ISSN 2161-3303.
- ↑ 8.0 8.1 "ObsEva Announces UK MHRA Marketing Authorization for Yselty (linzagolix), an Oral GnRH Antagonist, for the Treatment of Uterine Fibroids" (Press release). ObsEva. 28 June 2022. Retrieved 24 August 2022 – via GlobeNewswire.
- ↑ "Eliminating Hormones With Orally Active Gonadotropin-releasing Hormone Antagonists". Clinical Obstetrics and Gynecology 64 (4): 837–849. December 2021. doi:10.1097/GRF.0000000000000664. PMID 34668887.
- ↑ 10.0 10.1 "Yselty: Pending EC decision". 16 December 2021. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/yselty. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
Further reading
- "Linzagolix: a new GnRH-antagonist under investigation for the treatment of endometriosis and uterine myomas". Expert Opinion on Investigational Drugs 30 (9): 903–911. September 2021. doi:10.1080/13543784.2021.1957830. PMID 34278887.
External links
- "Linzagolix". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/linzagolix.
- "Linzagolix choline". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/linzagolix%20choline.
 |
