Chemistry:Iron tetracarbonyl diiodide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4FeI2O4 | |
| Molar mass | 421.694 g·mol−1 |
| Appearance | black solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
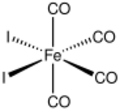
Iron tetracarbonyl diiodide is the inorganic compound with the formula FeI2(CO)4. The molecule features four carbonyl ligands and two iodides. It is a low-spin complex of ferrous iron. As confirmed by X-ray crystallography, the compound has cis stereochemistry.[1] It is a black solid that is soluble in dichloromethane and related organic solvents.
Preparation and reactions
It is prepared by the reaction of molecular iodine with iron pentacarbonyl, following a procedure first reported by Hieber and Wirschung in 1940:[2]
- Fe(CO)5 + I2 → FeI2(CO)4 + CO
Iron tetracarbonyl diiodide reacts with a variety of Lewis bases with displacement of one or two CO ligands.[3]
References
- ↑ Yu. V. Torubayev, A. A. Pasynskii, P. Mathur (2008). Koordinatsionnaya Khimiya 34: 812–816.
- ↑ Hieber, W.; Wirsching, A. (1940). "Über Metallcarbonyle. XXXII. Über Eisencarbonylhalogenide". Z. Anorg. Allg. Chem. 245: 35-58. doi:10.1002/zaac.19402450108.
- ↑ Li, Bin; Liu, Tianbiao; Popescu, Codrina V.; Bilko, Andrey; Darensbourg, Marcetta Y. (2009). "Synthesis and Mössbauer Characterization of Octahedral Iron(II) Carbonyl Complexes FeI2(CO)3L and FeI2(CO)2L2: Developing Models of the [Fe]-H2ase Active Site". Inorg. Chem. 48 (23): 11283–11289. doi:10.1021/ic9017882. PMID 19860458.
 |

