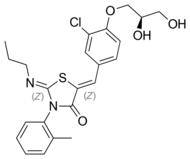
Chemistry:Ponesimod
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ponvory |
| Other names | ACT-128800 |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | 2 main metabolites |
| Elimination half-life | 31–34 hrs[7] |
| Excretion | Feces (57–80%, 26% unchanged), urine (10–18%)[8] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H25ClN2O4S |
| Molar mass | 460.97 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ponesimod, sold under the brand name Ponvory, is a medication for the treatment of multiple sclerosis.[4][9] It is a sphingosine-1-phosphate receptor modulator.[4]
The most common side effects include upper respiratory tract infection, hepatic transaminase elevation, and hypertension.[4][5][9]
Ponesimod was approved for medical use in the United States in March 2021,[4][9] and in the European Union in June 2021.[10]
Medical uses
Ponesimod is indicated for the treatment of relapsing forms of multiple sclerosis including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.[4][5]
Adverse effects
Common adverse effects in studies were temporary bradycardia (slow heartbeat), usually at the beginning of the treatment, dyspnoea (breathing difficulties), and increased liver enzymes (without symptoms). No significant increase of infections was observed under ponesimod therapy.[11] QT prolongation is detectable but was considered to be too low to be of clinical importance in a study.[12]
Mechanism of action
Like fingolimod, which is already approved for the treatment of multiple sclerosis, ponesimod blocks the sphingosine-1-phosphate receptor. This mechanism prevents lymphocytes (a type of white blood cells) from leaving lymph nodes.[11] Ponesimod is selective for subtype 1 of this receptor, S1P1.[13]
History
Clinical trials
In a 2009–2011 Phase II clinical trial including 464 multiple sclerosis patients, ponesimod treatment resulted in fewer new active brain lesions than placebo, measured during the course of 24 weeks.[11][14]
In a 2010–2012 Phase II clinical trial including 326 patients with psoriasis, 46 or 48% of patients (depending on dosage) had a reduction of at least 75% Psoriasis Area and Severity Index (PASI) score compared to placebo in 16 weeks.[11][15] The approval is already applied for in 2020.[16]
In a 2015–2019 Phase III randomised, double-blind clinical trial of 1133 adult patients with relapsing multiple sclerosis, those under ponesimod treatment showed a 30% reduction in annual relapse rate and a significantly reduced number of new inflammatory lesions on brain MRI by 56% compared to those taking teriflunomide.[17]
In October 2020, Janseen-Cilag International NV submitted an application for the modification of agreed pediatric investigation plan (PIP) to European Medicines Agency (including deferral and waiver criteria). This application was launched for the amendments of proposed changes against the European Medicines Agency’s decisions issued in November 2012 and April 2018. The approved procedure has already started in December 2022. To evaluate pharmacodynamics and pharmacokinetics efficacy of ponesimod in pediatric patients with relapsing-remitting multiple sclerosis (RRMS); a multicenter, randomized, double blind clinical study of duration of 108 weeks treatment for age group 10 to less than 18 years, is in progress. The clinical trial will end in November 2027.[18][19]
Society and culture
Legal status
In March 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorisation for the medicinal product Ponvory, intended for the treatment of active relapsing forms of multiple sclerosis.[20] The applicant for this medicinal product is Janssen-Cilag International N.V.[20] Ponesimod was approved for medical use in the European Union in May 2021.[5]
References
- ↑ 1.0 1.1 "Ponvory APMDS". 24 March 2022. https://www.tga.gov.au/resources/auspmd/ponvory.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". 21 December 2022. https://www.tga.gov.au/resources/resource/guidance/updates-prescribing-medicines-pregnancy-database.
- ↑ "Summary Basis of Decision (SBD) for Ponvory". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00546&lang=en.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Ponvory- ponesimod tablet, film coated Ponvory- ponesimod kit". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5be69660-738c-4db7-b0d4-517aa873dc41.
- ↑ 5.0 5.1 5.2 5.3 "Ponvory EPAR". 24 March 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/ponvory.
- ↑ "Ponvory Product information". https://ec.europa.eu/health/documents/community-register/html/h1550.htm.
- ↑ "Multiple-dose tolerability, pharmacokinetics, and pharmacodynamics of ponesimod, an S1P1 receptor modulator: favorable impact of dose up-titration". Journal of Clinical Pharmacology 54 (2): 179–188. February 2014. doi:10.1002/jcph.244. PMID 24408162.
- ↑ "Mass balance, pharmacokinetics and metabolism of the selective S1P1 receptor modulator ponesimod in humans". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 45 (2): 139–149. February 2015. doi:10.3109/00498254.2014.955832. PMID 25188442.
- ↑ 9.0 9.1 9.2 "Janssen Announces U.S. FDA Approval of Ponvory (ponesimod), an Oral Treatment for Adults with Relapsing Multiple Sclerosis Proven Superior to Aubagio (teriflunomide) in Reducing Annual Relapses and Brain Lesions" (Press release). Janssen. 19 March 2021. Archived from the original on 19 March 2021. Retrieved 19 March 2021 – via PR Newswire.
- ↑ "European Medicines Agency Approval of Ponesimod". 24 March 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/ponvory.
- ↑ 11.0 11.1 11.2 11.3 "Neue Wirkstoffe – Ponesimod" (in German). Österreichische Apothekerzeitung (20/2014): 42. 29 September 2014.
- ↑ "Effect of ponesimod, a selective S1P1 receptor modulator, on the QT interval in healthy individuals". Basic & Clinical Pharmacology & Toxicology 116 (5): 429–437. May 2015. doi:10.1111/bcpt.12336. PMID 25287214.
- ↑ "Ponesimod". Actelion. http://www1.actelion.com/sites/en/scientists/development-pipeline/phase-2/ponesimod.page.
- ↑ "Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial". Journal of Neurology, Neurosurgery, and Psychiatry 85 (11): 1198–1208. November 2014. doi:10.1136/jnnp-2013-307282. PMID 24659797.
- ↑ "Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial". Lancet 384 (9959): 2036–2045. December 2014. doi:10.1016/S0140-6736(14)60803-5. PMID 25127208.
- ↑ "Ozanimod bei schubförmiger MS zugelassen" (in de). 6 July 2020. https://www.amsel.de/multiple-sklerose-news/medizin/ozanimod-bei-schubfoermiger-ms-zugelassen/.
- ↑ "Europe Approves Ponvory Ponesimod for Multiple Sclerosis". https://www.europeanpharmaceuticalreview.com/news/155150/europe-approves-ponvory-ponesimod-for-multiple-sclerosis/.
- ↑ EMA (17 September 2018). "EMEA-000798-PIP01-09-M03" (in en). https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-000798-pip01-09-m03.
- ↑ "Children with multiple sclerosis should not become therapeutic hostages". Therapeutic Advances in Neurological Disorders 9 (5): 389–395. September 2016. doi:10.1177/1756285616656592. PMID 27582894.
- ↑ 20.0 20.1 "Ponvory: Pending EC decision". 25 March 2021. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/ponvory.
 |
