Chemistry:Etrasimod
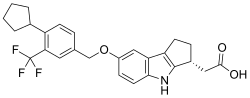 Skeletal formula of etrasimod | |
| Clinical data | |
|---|---|
| Trade names | Velsipity |
| Other names | APD334, APD-334 |
| License data | |
| Routes of administration | By mouth |
| Drug class | Sphingosine-1-phosphate receptor modulator |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 97.9% |
| Metabolism | Liver (CYP2C8, 2C9, 3A4) |
| Elimination half-life | 30 hours |
| Excretion | Feces (82%), kidneys (5%) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H26F3NO3 |
| Molar mass | 457.493 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etrasimod, sold under the brand name Velsipity, is a medication that is used for the treatment of ulcerative colitis (UC).[1] It is a selective sphingosine-1-phosphate (S1P) receptor modulator that modifies the activity of the immune system.[1] It is taken by mouth.[1]
Etrasimod was discovered by Arena Pharmaceuticals, with subsequent development by Pfizer.[2]
Medical uses
Etrasimod is used for the treatment of moderate to severe ulcerative colitis.[1]
Mechanism of action
It works by causing T cells to become trapped in the lymph nodes, preventing them from entering the bloodstream, from where they would travel to other tissues in the body and mediate inflammation.[3][4][5][6][7][8]
Society and culture
Legal status
Velsipity was approved by the US Food and Drug Administration (FDA) in October 2023.[1][9][10]
In December 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Velsipity, intended for the treatment of ulcerative colitis.[11] The applicant for this medicinal product is Pfizer Europe MA EEIG.[11]
Names
Etrasimod is the international nonproprietary name.[12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Pfizer (12 October 2023). "Velsipity (etrasimod) tablets, for oral use". U.S. Food and Drug Administration (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216956s000lbl.pdf.
- ↑ "Pfizer tosses newly acquired meds out of the Arena". Fierce Biotech. 2 May 2023. https://www.fiercebiotech.com/biotech/pfizer-tosses-newly-acquired-meds-out-arena-part-midstage-cleaning.
- ↑ "The sphingosine-1-phosphate receptor agonist etrasimod in ulcerative colitis". Lancet 401 (10383): 1132–1133. April 2023. doi:10.1016/S0140-6736(23)00228-3. PMID 36871570.
- ↑ "Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies". Lancet 401 (10383): 1159–1171. April 2023. doi:10.1016/S0140-6736(23)00061-2. PMID 36871574.
- ↑ "Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine". Biomedicines 10 (7): 1735. July 2022. doi:10.3390/biomedicines10071735. PMID 35885040.
- ↑ "Modulation of sphingosine-1-phosphate in ulcerative colitis". Expert Opinion on Biological Therapy 20 (4): 413–420. April 2020. doi:10.1080/14712598.2020.1732919. PMID 32093531.
- ↑ "The Selective Sphingosine 1-Phosphate Receptor Modulator Etrasimod Regulates Lymphocyte Trafficking and Alleviates Experimental Colitis". The Journal of Pharmacology and Experimental Therapeutics 369 (3): 311–317. June 2019. doi:10.1124/jpet.118.254268. PMID 30872391.
- ↑ "Modulation of sphingosine-1-phosphate in inflammatory bowel disease". Autoimmunity Reviews 16 (5): 495–503. May 2017. doi:10.1016/j.autrev.2017.03.007. PMID 28279838.
- ↑ "FDA Approves New Drug for Ulcerative Colitis". Medscape. 13 October 2023. https://www.medscape.com/viewarticle/997350.
- ↑ "Velsipity (etrasimod) tablets for the treatment of moderately to severely active ulcerative colitis in adults". Approval Letter. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/216956Orig1s000ltr.pdf.
- ↑ 11.0 11.1 "Velsipity EPAR". 14 December 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/velsipity. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information 31 (3). 2017.
 |

