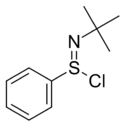
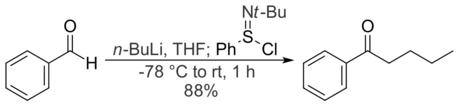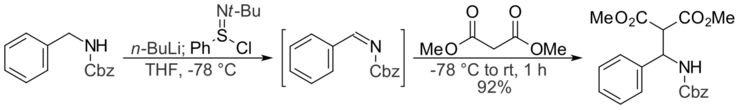Chemistry:N-tert-Butylbenzenesulfinimidoyl chloride
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N-tert-Butylbenzenesulfinimidoyl chloride | |||
| Systematic IUPAC name
[(1,1-Dimethylethyl)imino]chloro(phenyl)-λ4-sulfane | |||
| Other names
N-tert-Butylphenylsulfinimidoyl chloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 3261 | ||
| |||
| |||
| Properties | |||
| C10H14ClNS | |||
| Molar mass | 215.74 g·mol−1 | ||
| Appearance | Yellow to deep yellow-red crystals or powder | ||
| Melting point | 51 to 53 °C (124 to 127 °F; 324 to 326 K)[1] | ||
| Boiling point | 112 to 116 °C (234 to 241 °F; 385 to 389 K)[1] 0.5 mm Hg | ||
| Decomposes | |||
| Solubility in other solvents | Benzene, THF, DCM; slightly soluble in toluene | ||
| Hazards | |||
| Main hazards | Corrosive | ||
| Safety data sheet | TCI MSDS | ||
| GHS pictograms | 
| ||
| GHS Signal word | Danger | ||
| H290, H314 | |||
| P234, P260, P264, P280, P304+340, P301+330+331, P305+351+338, P303+361+353, P310, P363, P405 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
N-tert-Butylbenzenesulfinimidoyl chloride is a useful oxidant for organic synthesis reactions.[1] It is a good electrophile, and the sulfimide S=N bond can be attacked by nucleophiles, such as alkoxides, enolates, and amide ions. The nitrogen atom in the resulting intermediate is basic, and can abstract an α-hydrogen to create a new double bond.
Preparation
This reagent can be synthesized quickly and in near-quantitative yield by reacting phenyl thioacetate with tert-butyldichloroamine in hot benzene. After the reaction is complete, the product can be isolated as a yellow, moisture-sensitive solid by vacuum distillation.[2]
Mechanism
The first two steps in an oxidation reaction involving N-tert-butylbenzenesulfinimidoyl chloride are similar to a nucleophilic acyl substitution reaction. A nucleophile, such as an alkoxide (1), attacks the S=N bond in 2. The resulting intermediate (3) collapses and ejects chloride ion, which is a good leaving group. The resulting sulfimide has two resonance forms - 4a and 4b. Because of this, the nitrogen is basic, and via a five-membered ring transition state, it can abstract the hydrogen adjacent to the oxygen. This forms a new C=O bond and ejects a neutral sulfenamide (5), giving ketone 6 as the product. N-tert-Butylbenzenesulfinimidoyl chloride reacts with enolates, amides, and primary alkoxides by the same general mechanism.
The Swern oxidation, which converts primary and secondary alcohols to aldehydes and ketones, respectively, also uses a sulfur-containing compound (DMSO) as the oxidant and proceeds by a similar mechanism. In the Swern oxidation, elimination also occurs via a five-membered ring transition state, but the basic species is a sulfur ylide instead of a negatively charged nitrogen. Several other oxidation reactions also make use of DMSO as the oxidant and pass through a similar transition state (see #See also).
Reactions
Reacting an aldehyde with a Grignard reagent or organolithium and treating the resulting secondary alkoxide with N-tert-butylbenzenesulfinimidoyl chloride is a convenient one-pot reaction for converting aldehydes to ketones. While Grignards can be used for this reaction, organolithium compounds give higher yields, due to the higher reactivity of a lithium alkoxide compared to the corresponding magnesium salt. In some cases, an equivalent of DMPU, a Lewis base, will increase yields. For example, treating benzaldehyde with n-butyllithium and N-tert-butylbenzenesulfinimidoyl chloride in THF gives 1-phenyl-1-pentanone in good yield.[3]
N-tert-Butylbenzenesulfinimidoyl chloride can also be used to synthesize imines from amines. Imines synthesized in this fashion have been shown to undergo a one-pot Mannich reaction with 1,3-dicarbonyl compounds, such as malonate esters and 1,3-diketones. In this example, Cbz-protected benzylamine is deprotonated using n-butyllithium, then treated with N-tert-butylbenzenesulfinimidoyl chloride to form the protected imine. Dimethyl malonate acts as the nucleophile and reacts with the imine to give the final product, a Mannich base.[4]
See also
References
- ↑ 1.0 1.1 1.2 Matsuo, J.; Mukaiyama, T. (2001). "N-tert-Butylbenzenesulfinimidoyl Chloride". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X. ISBN 9780470842898. http://onlinelibrary.wiley.com/o/eros/articles/rn00306/frame.html. Retrieved 30 June 2012.
- ↑ Barrett, A. G. M.; Gray, A. A.; Hill, M. S.; Hitchcock, P. B.; Procopiou, P. A.; White, A. J. P. (2006). "Imino Sulfinamidines: Synthesis and Coordination Chemistry of a Novel Class of Chiral Bidentate Ligands". Inorganic Chemistry 45 (8): 3352–3358. doi:10.1021/ic052104m. PMID 16602794.
- ↑ Crawford, J. J.; Henderson, K. W.; Kerr, W. J. (2006). "Direct and Efficient One-Pot Preparation of Ketones from Aldehydes Using N-tert-Butylphenylsulfinimidoyl Chloride". Organic Letters 8 (22): 5073–5076. doi:10.1021/ol061903l. PMID 17048846. https://figshare.com/articles/Direct_and_Efficient_One_Pot_Preparation_of_Ketones_from_Aldehydes_Using_i_N_i_i_tert_i_Butylphenylsulfinimidoyl_Chloride/3051721.
- ↑ Matsuo, J.; Tanaki, Y.; Ishibashi, H. (2006). "Oxidative Mannich Reaction of N-Carbobenzyloxy Amines with 1,3-Dicarbonyl Compounds". Organic Letters 8 (19): 4371–4374. doi:10.1021/ol0618095. PMID 16956229.
External links
- N-tert-butylbenzenesulfinimidoyl chloride on Organic Chemistry Portal
 |