Chemistry:Nitrate radical
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitrate radical
| |||
| Systematic IUPAC name
Trioxidonitrogen(•) | |||
| Other names
Nitrooxy radical
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| 1573 | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| NO3 | |||
| Molar mass | 62.004 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
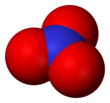
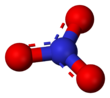
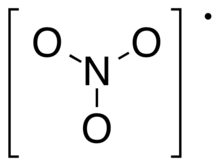
Nitrogen trioxide or nitrate radical is an oxide of nitrogen with formula NO3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.[1]
Like nitrogen dioxide NO2, it is a radical (a molecule with an unpaired valence electron), which makes it paramagnetic. It is the uncharged counterpart of the nitrate anion NO−3 and an isomer of the peroxynitrite radical OONO.[1]
Nitrogen trioxide is an important intermediate in reactions between atmospheric components, including the destruction of ozone.[1][2]
History
The existence of the NO3 radical was postulated in 1881-1882 by Hautefeuille and Chappuis to explain the absorption spectrum of air subjected to a silent electrical discharge.[1]
Structure and properties
The neutral NO3 molecule appears to be planar, with three-fold rotational symmetry (symmetry group D3h); or possibly a resonance between three Y-shaped molecules.[1]
The NO3 radical does not react directly with water, and is relatively unreactive towards closed-shell molecules, as opposed to isolated atoms and other radicals. It is decomposed by light of certain wavelengths into nitric oxide NO and oxygen O2.[1]
The absorption spectrum of NO3 has a broad band for light with wavelengths from about 500 to 680 nm, with three salient peaks in the visible at 590, 662, and 623 nm. Absorption in the range 640–680 nm does not lead to dissociation but to fluorescence: specifically, from about 605 to 800 nm following excitation at 604.4 nm, and from about 662 to 800 nm following excitation at 661.8 nm.[1] In water solution, another absorption band appears at about 330 nm (ultraviolet). An excited state NO*3 can be achieved by photons of wavelength less than 595 nm.[1]
Preparation
Nitrogen trioxide can be prepared in the gas phase by mixing nitrogen dioxide and ozone:[1]
- NO2 + O3 → NO3 + O2
This reaction can be performed also in the solid phase or water solutions, by irradiating frozen gas mixtures, flash photolysis and radiolysis of nitrate salts and nitric acid, and several other methods.[1]
Nitrogen trioxide is a product of the photolysis of dinitrogen pentoxide N2O5, chlorine nitrate ClONO2, and peroxynitric acid HO2NO2 and its salts.[1]
- N2O5 → NO2 + NO3
- 2 ClONO2 → Cl2 + 2 NO3
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 R. P. Wayne, I. Barnes, P. Biggs, J. P. Burrows, C. E. Canosa-Mas, J. Hjorth, G. Le Bras. G. K. Moortgat, D. Perner, G. Poulet, G. Restelli, and H. Sidebottom (1991): "The nitrate radical: Physics, chemistry, and the atmosphere". Atmospheric Environment. Part A. General Topics. volume 25, issue 1, pages 1-203. doi:10.1016/0960-1686(91)90192-A
- ↑ Richard A. Graham and Harold S. Johnston (1978): "The photochemistry of the nitrate radical and the kinetics of the nitrogen pentoxide-ozone system". Journal of Physical Chemistry, volume 82, issue 3, pages 254-268. doi:10.1021/j100492a002
 |