Chemistry:Chlorine nitrate
From HandWiki
| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chlorine nitrate | |||
| Systematic IUPAC name
Chloro nitrate | |||
| Other names
Nitryl hypochlorite
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| ClNO3 | |||
| Molar mass | 97.46 | ||
| Density | 1.65 g/cm3 | ||
| Melting point | −101 °C (−150 °F; 172 K)[1] | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
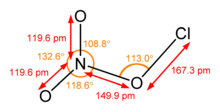
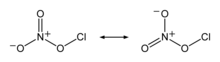
Chlorine nitrate, with chemical formula ClONO2 is an important atmospheric gas present in the stratosphere. It is an important sink of chlorine that contributes to the depletion of ozone.
Chemical properties
It explosively reacts with metals, metal chlorides, alcohols, ethers, and most organic materials. When it is heated to decomposition, it emits toxic fumes of Cl2 and NOx.[citation needed]
Synthesis and reactions
It can be produced by the reaction of dichlorine monoxide and dinitrogen pentoxide at 0 °C:[2]
- Cl2O + N2O5 → 2 ClONO2
or by the reaction:[3]
- ClF + HNO3 → HF + ClONO2
It can also react with alkenes:
- (CH3)2C=CH2 + ClONO2 → O2NOC(CH3)2CH2Cl
Chlorine nitrate reacts with metal chlorides:[4]
- 4 ClONO2 + TiCl4 → Ti(NO3)4 + 4 Cl2
References
- ↑ Obermeyer, Axel; Borrmann, Horst; Simon, Arndt (August 1995). "Crystal Structures and Bonding in NOCl, NO2Cl, and NO3Cl". Journal of the American Chemical Society 117 (30): 7887–7890. doi:10.1021/ja00135a006.
- ↑ Schmeisser, M.; Ruff, J. K. & Lustig, M. Chlorine(1) Nitrate Inorganic Syntheses, Wiley-Blackwell, https://doi.org/10.1002/9780470132401.ch34, 1967, 127-130
- ↑ Schack, Carl J. (1967-10-01). "New synthesis of chlorine nitrate". Inorganic Chemistry 6 (10): 1938–1939. doi:10.1021/ic50056a047. ISSN 0020-1669.
- ↑ 张青莲 (1991). 《无机化学丛书》第六卷:卤素、铜分族、锌分族. 北京: 科学出版社. pp. P338-341. ISBN 7-03-002238-6.
Salts and covalent derivatives of the nitrate ion
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | C | NO−3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |