Chemistry:Nitroauric acid
From HandWiki
Short description: Chemical compound
| |
| Names | |
|---|---|
| IUPAC name
Hydrogen tetranitratoaurate(III)[1]
| |
Other names
| |
| Identifiers | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| Properties | |
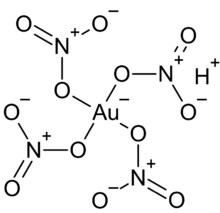
| HAu(NO 3) 4 | |
| Molar mass | 445.99 g/mol (anhydrous) 500.04 g/mol (trihydrate) |
| Appearance | Brown Crystals[1] |
| Density | 2.84 g/cm3[1] |
| Melting point | 72 °C (162 °F; 345 K)[3] |
| Soluble, decomposes[2] | |
| Solubility in nitric acid | Insoluble (0 °C) Soluble (30 °C) |
| Hazards | |
| Main hazards | Oxidizing |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H272, H302, H312, H315, H318, H332, H335 | |
| P210, P220, P221, P261, P280, P302+352, P304+340, P305+351+338, P332+313 | |
| NFPA 704 (fire diamond) | |
| Structure | |
| Octahedral | |
| Related compounds | |
Other anions
|
Chloroauric acid |
Other cations
|
Potassium tetranitratoaurate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitroauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO
3)
4 · 3H2O.[3] This compound is an intermediate in the process of extracting gold.[4] This compound is stable and soluble in aqueous nitric acid, but decomposes in water.[2]
Preparation
Nitroauric acid is prepared by the reaction of gold(III) hydroxide and concentrated nitric acid:[5]
- Au(OH)
3 + HNO
3 → HAu(NO
3)
4 + H
2O
Reactions
This compound reacts with potassium nitrate to form potassium tetranitratoaurate at 0 °C:[6]
- HAu(NO
3)
4 + KNO
3 → KAu(NO
3)
4 + HNO
3
Reference
- ↑ 1.0 1.1 1.2 "Gold Nitrate". ESPI Metals. https://www.espimetals.com/index.php/msds/574-gold-nitrate.
- ↑ 2.0 2.1 Arthur Messinger Comey (1896) (in en). A Dictionary of Chemical Solubilities Inorganic (2nd ed.). the University of Michigan: Macmillan & Company. p. 250.
- ↑ 3.0 3.1 A. Jamieson Walker (1924) (in en). The Alkali-metals and Their Congeners. the University of California: C. Griffin. p. 349.
- ↑ D. P. Graddon; H. Taube; A. G. Maddock (2017) (in en) (Ebook). An Introduction to Co-Ordination Chemistry (2nd ed.). Elsevier Science. p. 148. ISBN 9781483184111. https://www.google.com/books/edition/An_Introduction_to_Co_Ordination_Chemist/kSL-BAAAQBAJ?hl=en&gbpv=0.
- ↑ Harry Mann Gordin (1913) (in en). Elementary Chemistry (1 ed.). the University of Wisconsin - Madison: Medico-dental Publishing Company. p. 437.
- ↑ Ripan R., Chetyanu I. (1972). Inorganic chemistry. Chemistry of metals. 2. Moscow: World.

