Chemistry:Petrobactin
| |
| Names | |
|---|---|
| Preferred IUPAC name
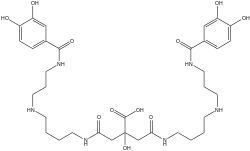
4-[4-[3-[(3,4-dihydroxybenzoyl)amino]propylamino]butylamino]-2-[2-[4-[3-[(3,4-dihydroxybenzoyl)amino]propylamino]butylamino]-2-oxoethyl]-2-hydroxy-4-oxobutanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C34H50N6O11 | |
| Molar mass | 718.79 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Petrobactin is a bis-catechol siderophore found in M. hydrocarbonoclasticus, A. macleodii, and the anthrax-producing B. anthracis.[1] Like other siderophores petrobactin is a highly specific iron(III) transport ligand, contributing to the marine microbial uptake of environmental iron.[1][2]
The iron-chelated petrobactin complex readily undergoes a photolytic oxidative decarboxylation due to its α-hydroxy carboxylate group, converting iron(III) to the more biologically useful iron(II).[3]

Biological function
Like other siderophores, petrobactin is secreted by an animal pathogenic bacterium. B. anthracis uses petrobactin to acquire iron from its host. Interestingly, while the 3,4-catecholate ends of petrobactin do not improve iron(III) affinity relative to hydroxamate ends, they speed up iron removal from human diferric transferrin.[4] Petrobactin in its ferric and iron-free forms is bound selectively by YclQ (an isogenic disruption mutant in the transporter encoded by the yclNOPQ operon in Bacillus subtilis), as is petrobactin's precursor protocatechuic acid and the ferric petrobactin photoproduct. The yclNOPQ operon is required for the utlization of petrobactin and yclNOPQ orthologs likely contribute to the pathogenicity of Bacilli.[5]
Biosynthesis
In B. anthracis, petrobactin is produced by a nonribosomal peptide synthetase independent siderophore (NIS) synthetase pathway.[6] The enzyme sequences used are anthrax siderophore biosynthesis (Asb) A through F, in alphabetical order. These gene clusters are identical to those used in M. hydrocarbonclasticus biosynthesis of petrobactin. In A. macleodii only the first three gene clusters, AsbA through AsbC, are identical to B. anthracis; then a longer AsbD and AsbF is next, followed by two hypothetical protein domains and a PepSY domain-containing gene. A. macleodii ends its sequence with AsbE.[1]
The biosynthesis of petrobactin in B. anthracis can progress in order AsbA-AsbB-AsbE-AsbE or AsbA-AsbE-AsbB-AsbE.[7]
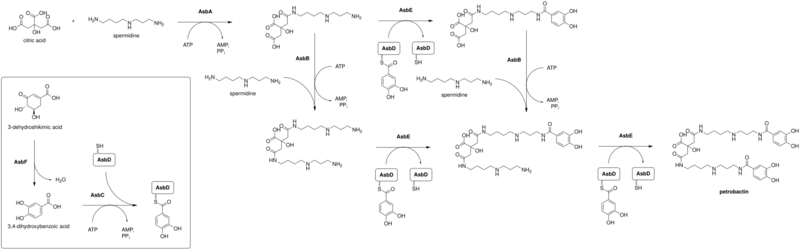
Abbreviations used: ATP, AMP, PPi.
If the enzymation reactions in this pathway proceed generally, in domains AsbA and AsbB the phosphorylation of a carboxylic acid forms an acylphosphate intermediate, which is then dephosphorylated by a primary amine in spermidine. In domain AsbE the lone pair of electrons on a primary amine allows for a nucleophilic attack on the electrophilic hydroxyl carbon. The sulfur on AsbE is protonated to form a thiol and the amide nitrogen is deprotonated.[8]
The dehydration of 3-dehydroshikimic acid might proceed as a modified, enzyme-catalyzed dienol benzene rearrangement and reduction, leading to aromatization of the ring.[9]
References
- ↑ 1.0 1.1 1.2 Manck, Lauren E.; Park, Jiwoon; Tully, Benjamin J.; Poire, Alfonso M.; Bundy, Randelle M.; Dupont, Christopher L.; Barbeau, Katherine A. (2021-08-02). "Petrobactin, a siderophore produced by Alteromonas, mediates community iron acquisition in the global ocean". The ISME Journal (Springer Science and Business Media LLC) 16 (2): 358–369. doi:10.1038/s41396-021-01065-y. ISSN 1751-7362. PMID 34341506.
- ↑ Rue, Eden L.; Bruland, Kenneth W. (1995). "Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method". Marine Chemistry (Elsevier BV) 50 (1–4): 117–138. doi:10.1016/0304-4203(95)00031-l. ISSN 0304-4203. Bibcode: 1995MarCh..50..117R.
- ↑ Barbeau, Katherine; Zhang, Guangping; Live, David H.; Butler, Alison (2001-12-27). "Petrobactin, a Photoreactive Siderophore Produced by the Oil-Degrading Marine Bacterium Marinobacter hydrocarbonoclasticus". Journal of the American Chemical Society (American Chemical Society (ACS)) 124 (3): 378–379. doi:10.1021/ja0119088. ISSN 0002-7863. PMID 11792199.
- ↑ Abergel, Rebecca J.; Zawadzka, Anna M.; Raymond, Kenneth N. (2008-01-26). "Petrobactin-Mediated Iron Transport in Pathogenic Bacteria: Coordination Chemistry of an Unusual 3,4-Catecholate/Citrate Siderophore". Journal of the American Chemical Society (American Chemical Society (ACS)) 130 (7): 2124–2125. doi:10.1021/ja077202g. ISSN 0002-7863. PMID 18220393.
- ↑ Zawadzka, Anna M.; Kim, Youngchang; Maltseva, Natalia; Nichiporuk, Rita; Fan, Yao; Joachimiak, Andrzej; Raymond, Kenneth N. (2009-12-22). "Characterization of a Bacillus subtilis transporter for petrobactin, an anthrax stealth siderophore". Proceedings of the National Academy of Sciences (Proceedings of the National Academy of Sciences) 106 (51): 21854–21859. doi:10.1073/pnas.0904793106. ISSN 0027-8424.
- ↑ Carroll, Cassandra S.; Moore, Margo M. (2018-06-04). "Ironing out siderophore biosynthesis: a review of non-ribosomal peptide synthetase (NRPS)-independent siderophore synthetases". Critical Reviews in Biochemistry and Molecular Biology (Informa UK Limited) 53 (4): 356–381. doi:10.1080/10409238.2018.1476449. ISSN 1040-9238. PMID 29863423.
- ↑ 7.0 7.1 Nusca, Tyler D.; Kim, Youngchang; Maltseva, Natalia; Lee, Jung Yeop; Eschenfeldt, William; Stols, Lucy; Schofield, Michael M.; Scaglione, Jamie B. et al. (2012). "Functional and Structural Analysis of the Siderophore Synthetase AsbB through Reconstitution of the Petrobactin Biosynthetic Pathway from Bacillus anthracis". Journal of Biological Chemistry (Elsevier BV) 287 (19): 16058–16072. doi:10.1074/jbc.m112.359349. ISSN 0021-9258. PMID 22408253.
- ↑ Petchey, Mark R.; Grogan, Gideon (2019-08-08). "Enzyme‐Catalysed Synthesis of Secondary and Tertiary Amides". Advanced Synthesis & Catalysis (Wiley) 361 (17): 3895–3914. doi:10.1002/adsc.201900694. ISSN 1615-4150. https://eprints.whiterose.ac.uk/150173/1/Accepted_Manuscript.docx.
- ↑ Gentles, Margaret Jevnik; Moss, Jane B.; Herzog, Hershel L.; Hershberg, E. B. (1958). "The Dienol-Benzene Rearrangement.1 Some Chemistry of 1,4-Androstadiene-3,17-dione". Journal of the American Chemical Society (American Chemical Society (ACS)) 80 (14): 3702–3705. doi:10.1021/ja01547a058. ISSN 0002-7863.
 |