Chemistry:Pracinostat
| |
| Names | |
|---|---|
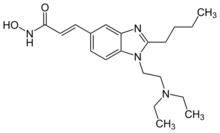
| Preferred IUPAC name
(2E)-3-{2-Butyl-1-[2-(diethylamino)ethyl]-1H-1,3-benzimidazol-5-yl}-N-hydroxyprop-2-enamide | |
| Other names
Pracinostat
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H30N4O2 | |
| Molar mass | 358.486 g·mol−1 |
| Density | 1.1±0.1 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pracinostat (SB939) is an orally bioavailable, small-molecule histone deacetylase (HDAC) inhibitor based on hydroxamic acid with potential anti-tumor activity characterized by favorable physicochemical, pharmaceutical, and pharmacokinetic properties.
Activity
Pracinostat selectively inhibits HDAC class I, II, IV without class III and HDAC6 in class IIb,[1] but has no effect on other Zn-binding enzymes, receptors, and ion channels. It accumulates in tumor cells and exerts a continuous inhibition to histone deacetylase, resulting in acetylated histones accumulation, chromatin remodeling, tumor suppressor genes transcription, and ultimately, apoptosis of tumor cells.[2]
Clinical medication
Clinical studies suggests that pracinostat has potential best pharmacokinetic properties when compared to other oral HDAC inhibitors.[3] In March 2014, pracinostat has granted Orphan Drug for acute myelocytic leukemia (AML) and for the treatment of T-cell lymphoma by the Food and Drug Administration.
References
- ↑ "In vitro enzyme activity of SB939 and SAHA". 22 Aug 2014. http://www.selleckchem.com/products/SB939.html.
- ↑ Novotny-Diermayr, V.; Hart, S.; Goh, K. C.; Cheong, A.; Ong, L-C; Hentze, H.; Pasha, M. K.; Jayaraman, R. et al. (2012). "The oral HDAC inhibitor pracinostat (SB939) is efficacious and synergistic with the JAK2 inhibitor pacritinib (SB1518) in preclinical models of AML". Blood Cancer Journal 2 (5): e69–. doi:10.1038/bcj.2012.14. PMID 22829971.
- ↑ Veronica Novotny-Diermayr (March 9, 2010). "SB939, a Novel Potent and Orally Active Histone Deacetylase Inhibitor with High Tumor Exposure and Efficacy in Mouse Models of Colorectal Cancer". Mol Cancer Ther 9 (3): 642–652. doi:10.1158/1535-7163.MCT-09-0689. PMID 20197387. http://mct.aacrjournals.org/content/9/3/642.long.
 |
