Chemistry:Allyl mercaptan
From HandWiki
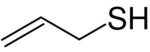
| |
| Names | |
|---|---|
| Preferred IUPAC name
Prop-2-ene-1-thiol | |
| Other names
2-Propene-1-thiol
Allyl thiol 3-Mercaptopropene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H6S | |
| Molar mass | 74.14 g·mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H302, H319, H332 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+312, P303+361+353, P304+312, P304+340, P305+351+338, P312, P330, P337+313, P370+378, P403+235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Allyl mercaptan (AM) is a small molecule allyl derivative and an organosulfur compound derived from garlic and a few other genus Allium plants. Its formula is C3H6S. It has been shown to be the most effective HDAC inhibitor of known garlic-derived organosulfur compounds and their metabolites.[1]
References
- ↑ Rajendran, Praveen; Ho, Emily; Williams, David E; Dashwood, Roderick H (2011). "Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells". Clinical Epigenetics 3 (1): 4. doi:10.1186/1868-7083-3-4. ISSN 1868-7083. PMID 22247744.
 |


