Chemistry:Parthenolide
From HandWiki
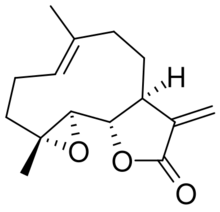
| |
| Names | |
|---|---|
| IUPAC name
(1aR,4E,7aS,10aS,10bR)-2,3,6,7,7a,8,10a,10b-octahydro-1a,5-dimethyl-8-methylene-oxireno[9,10]cyclodeca[1,2-b]furan-9(1aH)-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C15H20O3 | |
| Molar mass | 248.322 g·mol−1 |
| Melting point | 113 to 115 °C (235 to 239 °F; 386 to 388 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Parthenolide is a sesquiterpene lactone of the germacranolide class which occurs naturally in the plant feverfew (Tanacetum parthenium), after which it is named, and in the closely related tansy (Tanacetum vulgare).[1] It is found in highest concentration in the flowers and fruit. Parthenolide's molecular structure depiction is often incorrect regarding the stereochemistry of the epoxide, although X-ray single crystal structures are available.[2][3]
Lack of solubility in water and bioavailability limits the potential of parthenolide as a drug.
In vitro research
Parthenolide has a variety of reported in vitro biological activities, including:
- Inhibition of HDAC1 protein without affecting other class I/II HDACs, which leads to sustained DNA damage response in certain cells (required for apoptosis).[4]
- Modulation of the NF-κB-mediated inflammatory responses in experimental atherosclerosis.[5]
- Inducing apoptosis in acute myelogenous leukemia (AML) cells, leaving normal bone marrow cells relatively unscathed. Moreover, the compound may get at the root of the disease because it also kills stem cells that give rise to AML.[6]
- Activity against Leishmania amazonensis.[7]
- Microtubule-interfering activity.[8]
- Agonist of the adiponectin receptor 2 (AdipoR2).[9]
- Inhibition of mammalian thioredoxin reductase [10]
References
- ↑ Onozato, Thelma; Nakamura, Celso Vataru; Garcia Cortez, Diógenes Aparício; Dias Filho, Benedito Prado; Ueda-Nakamura, Tânia (2009). "Tanacetum vulgare: antiherpes virus activity of crude extract and the purified compound parthenolide". Phytother Res 23 (6): 791–6. doi:10.1002/ptr.2638. PMID 19152371.
- ↑ Quick, Andrew; Rogers, Donald (1976). "Crystal and molecular structure of parthenolide [4,5-epoxygermacra-1(10),11(13)-dien-12,6-olactone"] (in en). Journal of the Chemical Society, Perkin Transactions 2 (4): 465. doi:10.1039/p29760000465. ISSN 0300-9580. http://xlink.rsc.org/?DOI=p29760000465.
- ↑ Long, Jing; Ding, Ya-Hui; Wang, Pan-Pan; Zhang, Quan; Chen, Yue (2013-10-18). "Protection-Group-Free Semisyntheses of Parthenolide and Its Cyclopropyl Analogue" (in en). The Journal of Organic Chemistry 78 (20): 10512–10518. doi:10.1021/jo401606q. ISSN 0022-3263. PMID 24047483. http://pubs.acs.org/doi/10.1021/jo401606q.
- ↑ "Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells". Clinical Epigenetics 3 (1): 4. 2011. doi:10.1186/1868-7083-3-4. PMID 22247744.
- ↑ "Parthenolide modulates the NF-kappaB-mediated inflammatory responses in experimental atherosclerosis". Arteriosclerosis, Thrombosis, and Vascular Biology 26 (8): 1864–70. August 2006. doi:10.1161/01.ATV.0000229659.94020.53. PMID 16741149.
- ↑ "The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells". Blood 105 (11): 4163–9. June 2005. doi:10.1182/blood-2004-10-4135. PMID 15687234.
- ↑ "Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium". Antimicrobial Agents and Chemotherapy 49 (1): 176–82. January 2005. doi:10.1128/AAC.49.11.176-182.2005. PMID 15616293.
- ↑ "Microtubule-interfering activity of parthenolide". Chemico-Biological Interactions 149 (2–3): 165–73. October 2004. doi:10.1016/j.cbi.2004.07.005. PMID 15501437.
- ↑ "Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay". PLOS ONE 8 (5): e63354. 2013. doi:10.1371/journal.pone.0063354. PMID 23691032. Bibcode: 2013PLoSO...863354S.
- ↑ "Targeting Thioredoxin Reductase by Parthenolide Contributes to Inducing Apoptosis of HeLa Cells". The Journal of Biological Chemistry 291 (19): 10021–31. May 2016. doi:10.1074/jbc.M115.700591. PMID 27002142.
 |
