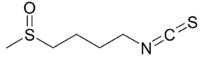
Chemistry:Sulforaphane
| |
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Isothiocyanato-4-(methanesulfinyl)butane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H11NOS2 | |
| Molar mass | 177.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulforaphane (sometimes sulphoraphane in British English) is a compound within the isothiocyanate group of organosulfur compounds.[1] It is produced when the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane upon damage to the plant (such as from chewing or chopping during food preparation), which allows the two compounds to mix and react.
Sulforaphane is present in cruciferous vegetables, such as broccoli, Brussels sprouts, and cabbage.[1]
Sulforaphane has two possible stereoisomers due to the presence of a stereogenic sulfur atom.[2]
The R-sulforaphane enantiomer occurs naturally, while the S-sulforaphane can be synthesized.[3]
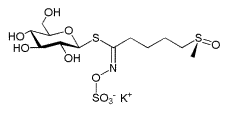 Glucoraphanin, the glucosinolate precursor to sulforaphane |
Occurrence and isolation
Sulforaphane occurs in broccoli sprouts, which, among cruciferous vegetables, have the highest concentration of glucoraphanin, the precursor to sulforaphane.[1][4] It is also found in cabbage, cauliflower, Brussels sprouts, bok choy, kale, collards, mustard greens, and watercress.[1]
Research
Although there has been some basic research on how sulforaphane might have effects in vivo, there is no clinical evidence that consuming cruciferous vegetables and sulforaphane affects the risk of cancer or any other disease, as of 2017.[1][5]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Isothiocyanates". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 April 2017. https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/isothiocyanates.
- ↑ "Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity". Molecules 27 (5): 1750. March 2022. doi:10.3390/molecules27051750. PMID 35268851.
- ↑ "Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway". npj Breast Cancer 8 (1): 40. March 2022. doi:10.1038/s41523-022-00402-4. PMID 35332167.
- ↑ "Sulforaphane: translational research from laboratory bench to clinic". Nutrition Reviews 71 (11): 709–726. November 2013. doi:10.1111/nure.12060. PMID 24147970.
- ↑ "Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: a systematic review of randomised trials". BJU International 117 Suppl 4 (Suppl 4): 17–34. April 2016. doi:10.1111/bju.13361. PMID 26898239.
 |
