Chemistry:Selone
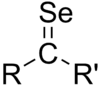
In chemistry, a selone (also known as a selenoketone) is the structural analog of a ketone where selenium replaces oxygen. Selenium-77 is one of the isotopes of selenium that is stable and naturally occurring, so selenoketone-containing chemicals can be analyzed by nuclear magnetic resonance spectroscopy (NMR). Selones can be used as chiral derivatizing agents for 77Se-NMR.[1] Chiral oxazolidineselones can be used for stereoselective control of aldol reactions, analogous to the Evans aldol reaction that uses oxazolidinones, which allows 77Se-NMR to be used to determine the diastereomeric ratio of the aldol product.[2]
Selenobenzophenone reversibly dimerizes. It is known to undergo cycloaddition with 1,3-dienes in a reaction similar to the Diels-Alder reaction.[3]
Further reading
References
- ↑ J. Peng; J. D. Odom; R. B. Dunlap; L. A. Silks III (1994). "Use of a selone chiral derivatizing agent for the absolute configurational assignment of stereogenic center". Tetrahedron: Asymmetry 5 (9): 1627–1630. doi:10.1016/0957-4166(94)80066-9. https://zenodo.org/record/1258653.
- ↑ L. Silks et al. (2009). "Chiral N-Acetyl Selone-Promoted Aldol Reactions". Synthetic Communications 39 (4): 641–653. doi:10.1080/00397910802419706. https://zenodo.org/record/1234399.
- ↑ Erker, G.; Hock, R.; Krüger, C.; Werner, S.; Klärner, F. G.; Artschwager-Perl, U. (1990). "Synthesis and Cycloadditions of Monomeric Selenobenzophenone". Angewandte Chemie International Edition in English 29 (9): 1067. doi:10.1002/anie.199010671.
 |
