Medicine:Dual-energy X-ray absorptiometry
| Dual-energy X-ray absorptiometry | |
|---|---|
 | |
| OPS-301 code | 3-900 |
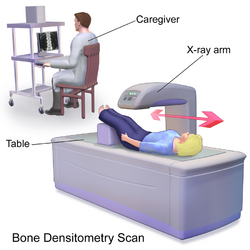
Dual-energy X-ray absorptiometry (DXA, or DEXA[1]) is a means of measuring bone mineral density (BMD) using spectral imaging. Two X-ray beams, with different energy levels, are aimed at the patient's bones. When soft tissue absorption is subtracted out, the bone mineral density (BMD) can be determined from the absorption of each beam by bone. Dual-energy X-ray absorptiometry is the most widely used and most thoroughly studied bone density measurement technology.
The DXA scan is typically used to diagnose and follow osteoporosis, as contrasted to the nuclear bone scan, which is sensitive to certain metabolic diseases of bones in which bones are attempting to heal from infections, fractures, or tumors. It is also sometimes used to assess body composition.
Physics
Soft tissue and bone have different attenuation coefficients to X-rays. A single X-ray beam passing through the body will be attenuated by both soft tissue and bone, and it is not possible to determine, from a single beam, how much attenuation was attributable to the bone. However, the attenuation coefficients vary with the energy of the X-rays, and, crucially, the ratio of the attenuation coefficients also varies. DXA uses two energies of X-ray. The difference in total absorption between the two can be used, by suitable weighting, to subtract out the absorption by soft tissue, leaving just the absorption by bone, which is related to bone density.
One type of DXA scanner uses a cerium filter with a tube voltage of 80 kV, resulting in effective photon energies of about 40 and 70 keV.[2] There is also a DXA scanner type using a samarium filter with a tube voltage of 100 kV, resulting in effective energies of 47 and 80 keV.[2] Also, the tube voltage can be continuously switched between a low (for example 70 kV) and high (for example 140 kV) value in synchronism with the frequency of the electrical mains, resulting in effective energies alternating between 45 and 100 keV.[2]
The combination of dual X-ray absorptiometry and laser uses the laser to measure the thickness of the region scanned, allowing for varying proportions of lean soft tissue and adipose tissue within the soft tissue to be controlled for and improving the accuracy.
Bone density measurement
Indications
The U.S. Preventive Services Task Force recommends that women over the age of 65 should get a DXA scan.[3] The date at which men should be tested is uncertain[3] but some sources recommend age 70.[4] At risk women should consider getting a scan when their risk is equal to that of a normal 65-year-old woman.
A person's risk can be measured using the University of Sheffield's FRAX calculator, which includes many different clinical risk factors including prior fragility fracture, use of glucocorticoids, heavy smoking, excess alcohol intake, rheumatoid arthritis, history of parental hip fracture, chronic renal and liver disease, chronic respiratory disease, long-term use of phenobarbital or phenytoin, celiac disease, inflammatory bowel disease, and other risks.[3]
Scoring
The World Health Organization has defined the following categories based on bone density in white women:
| Severe (established) osteoporosis | A T-score more than -2.5 standard deviations below the young adult female reference mean in the presence of one or more fragility fractures. |
Bone densities are often given to patients as a T score or a Z score. A T score tells the patient what their bone mineral density is in comparison to a young adult of the same gender with peak bone mineral density. A normal T score is -1.0 and above, low bone density is between -1.0 and -2.5, and osteoporosis is -2.5 and lower. A Z score is just a comparison of what a patient's bone mineral density is in comparison to the average bone mineral density of a male or female of their age and weight.
The WHO committee did not have enough data to create definitions for men or other ethnic groups.[5]
Special considerations are involved in the use of DXA to assess bone mass in children. Specifically, comparing the bone mineral density of children to the reference data of adults (to calculate a T-score) will underestimate the BMD of children, because children have less bone mass than fully developed adults. This would lead to an over-diagnosis of osteopenia for children. To avoid an overestimation of bone mineral deficits, BMD scores are commonly compared to reference data for the same gender and age (by calculating a Z-score).
Also, there are other variables in addition to age that are suggested to confound the interpretation of BMD as measured by DXA. One important confounding variable is bone size. DXA has been shown to overestimate the bone mineral density of taller subjects and underestimate the bone mineral density of smaller subjects. This error is due to the way by which DXA calculates BMD. In DXA, bone mineral content (measured as the attenuation of the X-ray by the bones being scanned) is divided by the area (also measured by the machine) of the site being scanned.
Because DXA calculates BMD using area (aBMD: areal Bone Mineral Density), it is not an accurate measurement of true bone mineral density, which is mass divided by a volume. In order to distinguish DXA BMD from volumetric bone-mineral density, researchers sometimes refer to DXA BMD as an areal bone mineral density (aBMD). The confounding effect of differences in bone size is due to the missing depth value in the calculation of bone mineral density. Despite DXA technology's problems with estimating volume, it is still a fairly accurate measure of bone mineral content. Methods to correct for this shortcoming include the calculation of a volume that is approximated from the projected area measure by DXA. DXA BMD results adjusted in this manner are referred to as the bone mineral apparent density (BMAD) and are a ratio of the bone mineral content versus a cuboidal estimation of the volume of bone. Like the results for aBMD, BMAD results do not accurately represent true bone mineral density, since they use approximations of the bone's volume. BMAD is used primarily for research purposes and is not yet used in clinical settings.
Other imaging technologies such as quantitative computed tomography (QCT) are capable of measuring the bone's volume, and are, therefore, not susceptible to the confounding effect of bone-size in the way that DXA results are susceptible.
It is important for patients to get repeat BMD measurements done on the same machine each time, or at least a machine from the same manufacturer. Error between machines, or trying to convert measurements from one manufacturer's standard to another can introduce errors large enough to wipe out the sensitivity of the measurements.[citation needed]
DXA results need to be adjusted if the patient is taking strontium supplements.[6][better source needed][7]
DXA can also used to measure trabecular bone score.
Current clinical practice in pediatrics
DXA is, by far, the most widely used technique for bone mineral density measurements, since it is considered to be cheap, accessible, easy to use, and able to provide an accurate estimation of bone mineral density in adults.[8]
The official position of the International Society for Clinical Densitometry (ISCD) is that a patient may be tested for BMD if they have a condition that could precipitate bone loss, is going to be prescribed pharmaceuticals known to cause bone loss, or is being treated and needs to be monitored. The ISCD states that there is no clearly understood correlation between BMD and the risk of a child's sustaining a fracture; the diagnosis of osteoporosis in children cannot be made using the basis of a densitometry criteria. T-scores are prohibited with children and should not even appear on DXA reports. Thus, the WHO classification of osteoporosis and osteopenia in adults cannot be applied to children, but Z-scores can be used to assist diagnosis.[9]
Some clinics may routinely carry out DXA scans on pediatric patients with conditions such as nutritional rickets, lupus, and Turner syndrome.[10] DXA has been demonstrated to measure skeletal maturity[11] and body fat composition[12] and has been used to evaluate the effects of pharmaceutical therapy.[13] It may also aid pediatricians in diagnosing and monitoring treatment of disorders of bone mass acquisition in childhood.[14]
However, it seems that DXA is still in its early days in pediatrics, and there are widely acknowledged limitations and disadvantages with DXA. A view exists[15] that DXA scans for diagnostic purposes should not even be performed outside specialist centers, and, if a scan is done outside one of these centers, it should not be interpreted without consultation with an expert in the field.[15] Furthermore, most of the pharmaceuticals given to adults with low bone mass can be given to children only in strictly monitored clinical trials.
Whole-body calcium measured by DXA has been validated in adults using in-vivo neutron activation of total body calcium[16][17] but this is not suitable for paediatric subjects and studies have been carried out on paediatric-sized animals.[16][17]
Body composition measurement
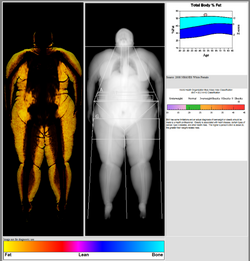
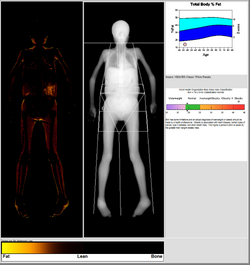
DXA scans can also be used to measure total body composition and fat content with a high degree of accuracy comparable to hydrostatic weighing with a few important caveats.[18][specify] From the DXA scans, a low resolution "fat shadow" image can also be generated, which gives an overall impression of fat distribution throughout the body[19] It has been suggested that, while very accurately measuring minerals and lean soft tissue (LST), DXA may provide skewed results due to its method of indirectly calculating fat mass by subtracting it from the LST and/or body cell mass (BCM) that DXA actually measures.[20]
DXA scans have been suggested as useful tools to diagnose conditions with an abnormal fat distribution, such as familial partial lipodystrophy.[21][22][19] They are also used to assess adiposity in children, especially to conduct clinical research.[23]
Radiation exposure
DXA uses X-rays to measure bone mineral density. The radiation dose of current DEXA systems is small,[24] as low as 0.001 mSv, much less than a standard chest or dental x-ray.[25][26] However, the dose delivered by older DEXA radiation sources (that used radioisotopes rather than x-ray generators) could be as high as 35 mGy,[27][28][29] considered a significant dose by radiological health standards.
miliSieverts and miliGrays are not compatible measurements.
Regulation
United States
The quality of DXA operators varies widely. DXA is not regulated like other radiation-based imaging techniques because of its low dosage. Each US state has a different policy as to what certifications are needed to operate a DXA machine. California , for example, requires coursework and a state-run test, whereas Maryland has no requirements for DXA technicians. Many states require a training course and certificate from the International Society of Clinical Densitometry (ISCD).
Australia
In Australia, regulation differs according to the applicable state or territory. For example, in Victoria, an individual performing DXA scans is required to completed a recognised course in safe use of bone mineral densitometers.[30] In NSW and QLD a DXA technician only requires prior study in science, nursing or other related undergraduate study. The Environmental Protection Agency (EPA) oversees licensing of technicians, however, this is far from rigorous and regulation is non-existent.
References
- ↑ "Bone mineral density test". U.S. National Library of Medicine. https://medlineplus.gov/ency/article/007197.htm.
- ↑ 2.0 2.1 2.2 "Physical Principles and Measurement Accuracy of Bone Densitometry". https://nos.org.uk/media/1725/02_physical_principles.pdf.
- ↑ 3.0 3.1 3.2 "Screening for Osteoporosis". uspreventiveservicestaskforce.org. U.S. Preventive Services Task Force. January 2011. http://www.uspreventiveservicestaskforce.org/uspstf/uspsoste.htm.
- ↑ American Academy of Family Physicians, "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation (American Academy of Family Physicians), http://choosingwisely.org/wp-content/uploads/2012/04/5things_12_factsheet_Amer_Acad_Fam_Phys.pdf, retrieved August 14, 2012
- ↑ "Bone densitometry". Courses.washington.edu. http://courses.washington.edu/bonephys/opbmd.html#tz.
- ↑ "Strontium dexa scan adjustments". Osteopenia3.com. http://www.osteopenia3.com/Strontium-dexa-scan.html.
- ↑ "Effect of bone strontium on BMD measurements". J Clin Densitom 10 (1): 34–8. 2007. doi:10.1016/j.jocd.2006.10.004. PMID 17289524.
- ↑ "Bone density in children: a review of the available techniques and indications". Eur J Radiol 26 (2): 177–82. January 1998. doi:10.1016/S0720-048X(97)00093-4. PMID 9518226.
- ↑ "2007 ISCD Official Positions". http://www.iscd.org/visitors/positions/OfficialPositionsText.cfm.
- ↑ "Pediatric DXA: technique and interpretation". Pediatr Radiol 37 (1): 21–31. January 2007. doi:10.1007/s00247-006-0153-y. PMID 16715219.
- ↑ "Evaluation of the possibility to assess bone age on the basis of DXA derived hand scans-preliminary results". Osteoporos Int 15 (4): 317–22. April 2004. doi:10.1007/s00198-003-1545-6. PMID 14615883.
- ↑ "Measurement of body fat using leg to leg bioimpedance". Arch. Dis. Child. 85 (3): 263–7. September 2001. doi:10.1136/adc.85.3.263. PMID 11517118.
- ↑ "Reduced bone density in children on long-term warfarin". Pediatr. Res. 57 (4): 578–81. April 2005. doi:10.1203/01.PDR.0000155943.07244.04. PMID 15695604.
- ↑ "Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults". Arch. Dis. Child. 87 (4): 341–7; discussion 341–7. October 2002. doi:10.1136/adc.87.4.341. PMID 12244017.
- ↑ 15.0 15.1 "First all-solid pediatric phantom for dual X-ray absorptiometry measurements in infants". J Clin Densitom 6 (1): 17–23. 2003. doi:10.1385/JCD:6:1:17. PMID 12665698.
- ↑ 16.0 16.1 "Reproducibility of pediatric whole body bone and body composition measures by dual-energy X-ray absorptiometry using the GE Lunar Prodigy". J Clin Densitom 8 (3): 298–304. 2005. doi:10.1385/JCD:8:3:298. PMID 16055960.
- ↑ 17.0 17.1 "Bone mineral in prepubertal children: gender and ethnicity". J. Bone Miner. Res. 15 (7): 1393–7. July 2000. doi:10.1359/jbmr.2000.15.7.1393. PMID 10893689.
- ↑ "Dual-energy x-ray absorptiometry-measured lean soft tissue mass: differing relation to body cell mass across the adult life span". J. Gerontol. A Biol. Sci. Med. Sci. 59 (8): 796–800. August 2004. doi:10.1093/gerona/59.8.B796. PMID 15345728.
- ↑ 19.0 19.1 ""Fat Shadows" From DXA for the Qualitative Assessment of Lipodystrophy: When a Picture Is Worth a Thousand Numbers". Diabetes Care 41 (10): 2255–2258. October 2018. doi:10.2337/dc18-0978. PMID 30237235.
- ↑ "Very-low-carbohydrate diets and preservation of muscle mass". Nutr Metab (Lond) 3: 9. January 2006. doi:10.1186/1743-7075-3-9. PMID 16448570.
- ↑ "Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort". Clin. Endocrinol. (Oxf) 86 (5): 698–707. May 2017. doi:10.1111/cen.13311. PMID 28199729.
- ↑ "Type 1 familial partial lipodystrophy: understanding the Köbberling syndrome". Endocrine 54 (2): 411–421. November 2016. doi:10.1007/s12020-016-1002-x. ISSN 1559-0100. PMID 27473102.
- ↑ "Identifying the best body mass index metric to assess adiposity change in children". Arch. Dis. Child. 99 (11): 1020–4. November 2014. doi:10.1136/archdischild-2013-305163. PMID 24842797.
- ↑ "Patient Safety - Radiation Dose in X-Ray and CT Exams". Radiological Society of North America. 2012-04-25. http://www.radiologyinfo.org/en/safety/index.cfm?pg=sfty_xray#part3.
- ↑ "Bone Densitometry (DEXA, DXA)". Radiological Society of North America. https://www.radiologyinfo.org/en/info.cfm?pg=dexa&bhcp=1.
- ↑ Radiology (ACR), Radiological Society of North America (RSNA) and American College of. "Patient Safety - Radiation Dose in X-Ray and CT Exams" (in en). https://www.radiologyinfo.org/en/info.cfm?pg=safety-xray.
- ↑ "Patient dose in dual x-ray absorptiometry". Osteoporos Int 4 (1): 11–5. January 1994. doi:10.1007/BF02352255. PMID 8148566.
- ↑ "Technical principles of dual energy x-ray absorptiometry". Semin Nucl Med 27 (3): 210–28. July 1997. doi:10.1016/S0001-2998(97)80025-6. PMID 9224663.
- ↑ "Radiation exposure in bone mineral density assessment". Appl Radiat Isot 50 (1): 215–36. January 1999. doi:10.1016/S0969-8043(98)00026-8. PMID 10028639.
- ↑ "Bone mineral densitometer operators". health.vic. https://www2.health.vic.gov.au/public-health/radiation/licensing/use-licences-employees/sector-specific-information/medical/bone-mineral-densitometer-operators.
External links
- Non-invasive testing of bone density explained
- Information for patients, from RSNA
- Bone Densitometry explained
es:Densitometría osea
 |