Physics:Chitosan nanoparticles
Chitosan-poly (acrylic acid) is a composite that has been increasingly used to create chitosan-poly(acrylic acid) nanoparticles.[1][2][3] More recently, various composite forms have come out with poly(acrylic acid) being synthesized with chitosan which is often used in a variety of drug delivery processes. Chitosan which already features strong biodegradability and biocompatibility nature can be merged with polyacrylic acid to create hybrid nanoparticles that allow for greater adhesion qualities as well as promote the biocompatibility and homeostasis nature of chitosan poly(acrylic acid) complex.[1] The synthesis of this material is essential in various applications and can allow for the creation of nanoparticles to facilitate a variety of dispersal and release behaviors and its ability to encapsulate a multitude of various drugs and particles.
Background
Research on nanoparticles and their chitosan nanoparticles grew in popularity in the early 1990s.[1][2][3] mainly due to its biodegradability and biocompatibility nature. Chitosan, due to its molecular structure, can be dissolved well within a variety of solvents and a variety of biologics, such as acids like formic and lactic acid.[3] Additionally, a benefit of chitosan is its ability to be greatly modified such as with other natural materials, synthetic materials, ligands, and even functionalized with various techniques.[2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18] Such an experience can be seen with the synthesis with poly-(acrylic acid) devices.[7][14][19] The addition of poly-(acrylic acid) can allow for an interaction to induce amphiphilicity and be spontaneously assembled.[7][14][19] This can be important due to the beneficial impact on its stimuli responsiveness and for large-scale use.[7][14][19]
Structure, properties, and synthesis
Chitosan
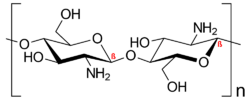
Chitosan is a polysaccharide that is derived from chitin that is composed of an alkaline deacetylated monomer of glucosamine and an acetylated monomor glucosamine and binding through β-1,4 glycosidic and hydrogen bonds.[2][3] The benefit of chitosan comes from their reactive groups such as -OH and -NH2.[11] Various mechanisms for chitosan exist, and various isolation techniques can be issued for the fabrication of chitosan nanoparticles.
Chitosan nanoparticle synthesis
There are various mechanisms for chitosan nanoparticle synthesis. These mechanisms include ionic gelation/polyelectrolyte complexation, emulsion droplet coalescence, emulsion solvent diffusion, reverse miscellisation, desolvation, emulsification cross-linking, nanoprecipitation, and spray-drying.[3][15]
Ionic gelation/polyelectrolyte complexation
Ionic gelation/polyelectrolyte complexation involves converting cationic chitosan solution with anionic tripolyphosphate and collecting precipitate in the form of nanoparticles.[3][20][21][22]
Emulsion droplet coalescence
Emulsion droplet coalescence involves the formulation of chitosan nanoparticles by creating two stable emulsions with liquid paraffin by adding one with a stabilizer and another with sodium hydroxide again containing a stabilizer. This mixture of the two emulsions can be used to form nanoparticles.[3][23]
Emulsion solvent diffusion
Emulsion solvent diffusion takes chitosan with stabilizer mixed in with an organic solvent such as methylene chloride/acetone that contains a drug that is hydrophilic and is diffused with acetone and chitosan nanoparticles are derived via centrifugation.[3][24]
Reverse miscellisation
Reverse miscellisation involves taking an organic solvent lipophilic surfactant and adding chitosan with a drug and cross-linker like glutaraldehyde. The nanoparticles are then extracted.[3][25]
Desolvation
Desolvation includes preparing chitosan solution and adding a precipitate with a stabilizing solution and precipitate such as acetone. Due to the insolubility of chitosan, the precipitate begins to form through the elimination of the liquid surrounding chitosan. A crosslinker such as glutaraldehyde can be added to formulate the nanoparticles[3][26]
Emulsification cross-linking
Chitosan-based solution is developed in the oil face and translated into stabilized liquid. A crosslinker such as glutaraldehyde can then be used to derive chitosan nanoparticles.[3][27]
Nanoprecipitation
Nanoprecipitation refers to using chitosan and dissolving it within a solvent and then having a pump to differentiate the dispersing phase and with tween 80, derive nanoparticles from the dispersing phase.[3][28]
Spray drying
Spray drying involves taking chitosan and adding it to the solvent acetic acid solution. The solution will then be atomized. These droplets will be mixed with drying gas and after further evaporation, nanoparticles can be derived[3][29]
Poly(acrylic acid)
Poly(acrylic acid) refers to acrylic acid that is polymerized. Poly(acrylic acid) is also known to have a neutral pH, have beneficial crosslinking properties, due to the charge properties of the side changes and poly(acrylic acid) being anionic[1][11][12][13][21][22] 1,11–13,21,22. Poly (acrylic acid) is known to have good biocompatibility with chitosan, particularly with the amine groups (-NH2)[30]
Chitosan-poly(acrylic acid) nanoparticles
An alternative method for the fabrication of chitosan nanoparticles includes the inclusion of polymerized groups of chitosan (Figure 2). This methodology can allow for the improvement of the chitosan cross-linking mechanism and improve overall drug release profiles for drugs such as amoxicillin and meloxicam.[1][31] Additionally, when poly (acrylic acid) is localized within the inner shell, overall drug encapsulation can be improved.[19][30]
Ionic gelation with radical polymerization
Ionic gelation with radical polymerization takes in a chitosan solution after through the addition of an acid monomer, the chitosan changes from the anion of an acrylic monomer. The nanoparticles are then derived after being self-settled overnight, and the unreacted monomer is removed. This is the main method for the formulation of poly (acrylic acid) based chitosan nanoparticles.[1][3][11][14]
Figure 2 Procedure way for the formation of chitosan poly(acrylic acid) nanoparticles. Adopted from Saberi et al, 2018.[1]
Applications
Biomedical applications
Biomedical applications of chitosan-based nanoparticles range from cancer treatment to regenerative medicine and tissue engineering to inflammatory diseases to diabetic treatment to the treatment of cerebral diseases, cardiovascular diseases, infectious diseases, and even for vaccine delivery.[3] Lung cancer, breast cancer, and colorectal cancer include the top 3 cancers in terms of frequency and are responsible for 1 out of 3 cancer cases and death burden worldwide.[32] Chitosan-based nanoparticles provide benefits to make targeted drug delivery systems for biomedical use and overall improve the potential of oral administration of drugs (Figure 3).[1][3][15][33]
Figure 3 Advantages of chitosan nanoparticles. Adopted from Sharifi-Rad et al, 2021.[32]
Drug delivery system
One of the main uses of chitosan-based nanoparticles involves drug delivery devices. The following are drugs delivered with chitosan-based nanoparticle: methotrexate, fucose-conjugated chitosan, 5-fluorouracil, doxorubicin, docetaxel, paclitaxel, propranolol-HCL, CyA, insulin, indomethacin, cefazolin, isoniazid, tetracycline, didanosine, isoniazid, rifampicin, folate, zaltoprofen, curcumin, cisplatin, camptothecin, bupivacaine, paclitaxel, prothionamide, hydrocortisone, albumin, ocimum gratissimum essential oil, triphosphate, RGD peptides and morphine.[3][32][33] The targeting system again ranges from various drug systems, with a primary focus on targeting cancer within specific organs such as lung or colorectal. The potential of poly(acrylic acid) and the addition has shown success in improvements of overall gene expression and protein delivery through the ability to modify pH sensitivity, modify chemosensitivity, and modify targeting.[2][10][14][15][17][18][19][22][26][28][29][30]
Drug encapsulating system
Another main use of chitosan-based nanoparticles involves the ability to withhold various drugs, organic compounds, and even inorganic analytes 5,8,9,11,12,23–25,28,32. These analytes include Fe3O4 (Figure 4).[3][5][9][11] A Fe3O4 based chitosan poly(acrylic acid) nanoparticle or nanosphere can have applications such as toxic metal uptake for direct use in drug delivery systems, treatment of tumors, magnetic separation of biomolecules, and even MRI contrast enhancement.[3][5][9][11]
Figure 4 Magnetic nanospheres with chitosan-poly(acrylic acid). Adopted from Feng et al, 2009.[9]
Limitations and future work
Overall continued improvement of stability, biocompatibility, degradability, and nontoxicity is needed to improve the viability.[1][3][15][33] Current limitations exist in routes of delivery, such as limited work in orally administered nanoparticles and drug delivery devices. Absorption should further be improved in chitosan poly(acrylic acid) nanoparticles for improved solubility for targeted drug delivery.[1][3][15][33] Additionally, further work in cell viability and cell proliferation is needed within these nanoparticles for use in tissue regeneration. Additionally, current limitations exist in fabrication techniques and large chain implementation due to possible difficulties in the synthesis of chitosan-based nanoparticles.[1][3][15][33]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Saberi, Javad; Ansari, Mojtaba; Ebrahimi Hoseinzadeh, Bahman; Kordestani, Soheila Salahshour; Naghib, Seyed Morteza (December 2018). "Chitosan-Polyacrylic Acid Hybrid Nanoparticles as Novel Tissue Adhesive: Synthesis and Characterization" (in en). Fibers and Polymers 19 (12): 2458–2464. doi:10.1007/s12221-018-8762-2. ISSN 1229-9197. http://link.springer.com/10.1007/s12221-018-8762-2.
- ↑ 2.0 2.1 2.2 2.3 2.4 Mohammed, Munawar; Syeda, Jaweria; Wasan, Kishor; Wasan, Ellen (2017-11-20). "An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery" (in en). Pharmaceutics 9 (4): 53. doi:10.3390/pharmaceutics9040053. ISSN 1999-4923. PMID 29156634.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 Naskar, Sweet; Sharma, Suraj; Kuotsu, Ketousetuo (February 2019). "Chitosan-based nanoparticles: An overview of biomedical applications and its preparation" (in en). Journal of Drug Delivery Science and Technology 49: 66–81. doi:10.1016/j.jddst.2018.10.022. https://linkinghub.elsevier.com/retrieve/pii/S1773224718306026.
- ↑ Risbud, Makarand V; Hardikar, Anandwardhan A; Bhat, Sujata V; Bhonde, Ramesh R (July 2000). "pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery" (in en). Journal of Controlled Release 68 (1): 23–30. doi:10.1016/S0168-3659(00)00208-X. PMID 10884576. https://linkinghub.elsevier.com/retrieve/pii/S016836590000208X.
- ↑ 5.0 5.1 5.2 Zhang, Sai; Zhou, YiFeng; Nie, WangYan; Song, LinYong (December 2012). "Preparation of Fe3O4/chitosan/poly(acrylic acid) composite particles and its application in adsorbing copper ion (II)" (in en). Cellulose 19 (6): 2081–2091. doi:10.1007/s10570-012-9783-4. ISSN 0969-0239. http://link.springer.com/10.1007/s10570-012-9783-4.
- ↑ Wang, Xue; Chen, Changjing; Huo, Da; Qian, Hanqing; Ding, Yin; Hu, Yong; Jiang, Xiqun (September 2012). "Synthesis of β-cyclodextrin modified chitosan–poly(acrylic acid) nanoparticles and use as drug carriers" (in en). Carbohydrate Polymers 90 (1): 361–369. doi:10.1016/j.carbpol.2012.05.052. PMID 24751053. https://linkinghub.elsevier.com/retrieve/pii/S0144861712005115.
- ↑ 7.0 7.1 7.2 7.3 Belbekhouche, S.; Mansour, O.; Carbonnier, B. (July 2018). "Promising sub-100 nm tailor made hollow chitosan/poly(acrylic acid) nanocapsules for antibiotic therapy" (in en). Journal of Colloid and Interface Science 522: 183–190. doi:10.1016/j.jcis.2018.03.061. PMID 29601960. https://linkinghub.elsevier.com/retrieve/pii/S0021979718303114.
- ↑ Yan, Han; Yang, Lingyun; Yang, Zhen; Yang, Hu; Li, Aimin; Cheng, Rongshi (August 2012). "Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions" (in en). Journal of Hazardous Materials 229-230: 371–380. doi:10.1016/j.jhazmat.2012.06.014. PMID 22749139. https://linkinghub.elsevier.com/retrieve/pii/S0304389412006346.
- ↑ 9.0 9.1 9.2 9.3 Feng, B.; Hong, R.Y.; Wu, Y.J.; Liu, G.H.; Zhong, L.H.; Zheng, Y.; Ding, J.M.; Wei, D.G. (April 2009). "Synthesis of monodisperse magnetite nanoparticles via chitosan–poly(acrylic acid) template and their application in MRI" (in en). Journal of Alloys and Compounds 473 (1–2): 356–362. doi:10.1016/j.jallcom.2008.05.094. https://linkinghub.elsevier.com/retrieve/pii/S0925838808008864.
- ↑ 10.0 10.1 Chen, Qi; Hu, Yong; Chen, Ying; Jiang, Xiqun; Yang, Yonghua (2005-10-20). "Microstructure Formation and Property of Chitosan-Poly(acrylic acid) Nanoparticles Prepared by Macromolecular Complex" (in en). Macromolecular Bioscience 5 (10): 993–1000. doi:10.1002/mabi.200500098. ISSN 1616-5187. PMID 16211548. https://onlinelibrary.wiley.com/doi/10.1002/mabi.200500098.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Guo, Liang; Liu, Guang; Hong, Ruo-Yu; Li, Hong-Zhong (2010-07-23). "Preparation and Characterization of Chitosan Poly(acrylic acid) Magnetic Microspheres" (in en). Marine Drugs 8 (7): 2212–2222. doi:10.3390/md8072212. ISSN 1660-3397. PMID 20714433.
- ↑ 12.0 12.1 Wang, Jian-Wen; Kuo, Yi-Ming (2008-02-15). "Preparation and adsorption properties of chitosan–poly(acrylic acid) nanoparticles for the removal of nickel ions" (in en). Journal of Applied Polymer Science 107 (4): 2333–2342. doi:10.1002/app.27247. https://onlinelibrary.wiley.com/doi/10.1002/app.27247.
- ↑ 13.0 13.1 Wu, Yan; Guo, Jia; Yang, Wuli; Wang, Changchun; Fu, Shoukuan (July 2006). "Preparation and characterization of chitosan–poly(acrylic acid) polymer magnetic microspheres" (in en). Polymer 47 (15): 5287–5294. doi:10.1016/j.polymer.2006.05.017. https://linkinghub.elsevier.com/retrieve/pii/S0032386106005878.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 Hu, Yong; Jiang, Xiqun; Ding, Yin; Ge, Haixiong; Yuan, Yuyan; Yang, Changzheng (August 2002). "Synthesis and characterization of chitosan–poly(acrylic acid) nanoparticles" (in en). Biomaterials 23 (15): 3193–3201. doi:10.1016/S0142-9612(02)00071-6. PMID 12102191. https://linkinghub.elsevier.com/retrieve/pii/S0142961202000716.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Zeng, Zhaowu (April 2011). "Recent advances of chitosan nanoparticles as drug carriers" (in en). International Journal of Nanomedicine 6: 765–774. doi:10.2147/IJN.S17296. ISSN 1178-2013. PMID 21589644.
- ↑ Yanat, Murat; Schroën, Karin (April 2021). "Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging" (in en). Reactive and Functional Polymers 161: 104849. doi:10.1016/j.reactfunctpolym.2021.104849. https://linkinghub.elsevier.com/retrieve/pii/S1381514821000419.
- ↑ 17.0 17.1 Park, Jae Hyung; Saravanakumar, Gurusamy; Kim, Kwangmeyung; Kwon, Ick Chan (January 2010). "Targeted delivery of low molecular drugs using chitosan and its derivatives" (in en). Advanced Drug Delivery Reviews 62 (1): 28–41. doi:10.1016/j.addr.2009.10.003. PMID 19874862. https://linkinghub.elsevier.com/retrieve/pii/S0169409X09003238.
- ↑ 18.0 18.1 Lai, Patrick; Daear, Weiam; Löbenberg, Raimar; Prenner, Elmar J. (June 2014). "Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly(d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate" (in en). Colloids and Surfaces B: Biointerfaces 118: 154–163. doi:10.1016/j.colsurfb.2014.03.017. PMID 24769392. https://linkinghub.elsevier.com/retrieve/pii/S0927776514001374.
- ↑ 19.0 19.1 19.2 19.3 19.4 Hu, Yong; Chen, Ying; Chen, Qi; Zhang, Leyang; Jiang, Xiqun; Yang, Changzheng (December 2005). "Synthesis and stimuli-responsive properties of chitosan/poly(acrylic acid) hollow nanospheres" (in en). Polymer 46 (26): 12703–12710. doi:10.1016/j.polymer.2005.10.110. https://linkinghub.elsevier.com/retrieve/pii/S0032386105015818.
- ↑ Alam, Sanjar; Mustafa, Gulam; Khan, Zeenat Iqbal; Islam, Fakhrul; Bhatnagar, Aseem; Ahmad, Farhan; Kumar (November 2012). "Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study" (in en). International Journal of Nanomedicine 7: 5705–5718. doi:10.2147/IJN.S35329. ISSN 1178-2013. PMID 23180965.
- ↑ 21.0 21.1 Pan, Yan; Li, Ying-jian; Zhao, Hui-ying; Zheng, Jun-min; Xu, Hui; Wei, Gang; Hao, Jin-song; Cui, Fu-de (December 2002). "Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo" (in en). International Journal of Pharmaceutics 249 (1–2): 139–147. doi:10.1016/S0378-5173(02)00486-6. PMID 12433442. https://linkinghub.elsevier.com/retrieve/pii/S0378517302004866.
- ↑ 22.0 22.1 22.2 Kawashima, Y.; Handa, T.; Kasai, A.; Takenaka, H.; Lin, S.Y.; Ando, Y. (March 1985). "Novel Method for the Preparation of Controlled-Release Theophylline Granules Coated with a Polyelectrolyte Complex of Sodium Polyphosphate-Chitosan" (in en). Journal of Pharmaceutical Sciences 74 (3): 264–268. doi:10.1002/jps.2600740308. PMID 4009432. https://linkinghub.elsevier.com/retrieve/pii/S0022354915466055.
- ↑ Flohr, H.; Breull, W. (September 1975). "Effect of etafenone on total and regional myocardial blood flow". Arzneimittel-Forschung 25 (9): 1400–1403. ISSN 0004-4172. PMID 23. https://pubmed.ncbi.nlm.nih.gov/23.
- ↑ Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. (May 1993). "Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D,L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior" (in en). Journal of Controlled Release 25 (1–2): 89–98. doi:10.1016/0168-3659(93)90097-O. https://linkinghub.elsevier.com/retrieve/pii/016836599390097O.
- ↑ Banerjee, Tanima; Mitra, Susmita; Kumar Singh, Ajay; Kumar Sharma, Rakesh; Maitra, Amarnath (August 2002). "Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles" (in en). International Journal of Pharmaceutics 243 (1–2): 93–105. doi:10.1016/S0378-5173(02)00267-3. PMID 12176298. https://linkinghub.elsevier.com/retrieve/pii/S0378517302002673.
- ↑ 26.0 26.1 Scherberger, R. R.; Kaess, H.; Brückner, S. (September 1975). "[Studies on the action of an anticholinergic agent in combination with a tranquilizer on gastric juice secretion in man"]. Arzneimittel-Forschung 25 (9): 1460–1463. ISSN 0004-4172. PMID 26. https://pubmed.ncbi.nlm.nih.gov/26.
- ↑ Conway, C. M. (August 1975). "Editorial: "Old lamps for new"". British Journal of Anaesthesia 47 (8): 811–812. doi:10.1093/bja/47.8.813. ISSN 0007-0912. PMID 27. https://pubmed.ncbi.nlm.nih.gov/27.
- ↑ 28.0 28.1 Luque-Alcaraz, A. G.; Lizardi-Mendoza, J.; Goycoolea, F. M.; Higuera-Ciapara, I.; Argüelles-Monal, W. (2016). "Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier" (in en). RSC Advances 6 (64): 59250–59256. doi:10.1039/C6RA06563E. ISSN 2046-2069. Bibcode: 2016RSCAd...659250L. http://xlink.rsc.org/?DOI=C6RA06563E.
- ↑ 29.0 29.1 Kulvanich, Poj; Sinsuebpol, Chutima; Chatchawalsaisin, Jittima (August 2013). "Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery" (in en). Drug Design, Development and Therapy 7: 861–873. doi:10.2147/DDDT.S47681. ISSN 1177-8881. PMID 24039397.
- ↑ 30.0 30.1 30.2 Hu, Yong; Ding, Yin; Ding, Dan; Sun, Mingjie; Zhang, Leyang; Jiang, Xiqun; Yang, Changzheng (2007-04-01). "Hollow Chitosan/Poly(acrylic acid) Nanospheres as Drug Carriers" (in en). Biomacromolecules 8 (4): 1069–1076. doi:10.1021/bm0608176. ISSN 1525-7797. PMID 17326676. https://pubs.acs.org/doi/10.1021/bm0608176.
- ↑ Wang, Yiming; Wang, Jie; Yuan, Zhenyu; Han, Haoya; Li, Tao; Li, Li; Guo, Xuhong (April 2017). "Chitosan cross-linked poly(acrylic acid) hydrogels: Drug release control and mechanism" (in en). Colloids and Surfaces B: Biointerfaces 152: 252–259. doi:10.1016/j.colsurfb.2017.01.008. PMID 28119220. https://linkinghub.elsevier.com/retrieve/pii/S0927776517300164.
- ↑ 32.0 32.1 32.2 Sharifi-Rad, Javad; Quispe, Cristina; Butnariu, Monica; Rotariu, Lia Sanda; Sytar, Oksana; Sestito, Simona; Rapposelli, Simona; Akram, Muhammad et al. (December 2021). "Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment" (in en). Cancer Cell International 21 (1): 318. doi:10.1186/s12935-021-02025-4. ISSN 1475-2867. PMID 34167552.
- ↑ 33.0 33.1 33.2 33.3 33.4 Garg, Unnati; Chauhan, Swati; Nagaich, Upendra; Jain, Neha (2019-06-01). "Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting" (in en). Advanced Pharmaceutical Bulletin 9 (2): 195–204. doi:10.15171/apb.2019.023. ISSN 2228-5881. PMID 31380245. PMC 6664124. https://apb.tbzmed.ac.ir/Abstract/apb-23086.
 |