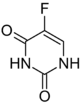
Chemistry:Fluorouracil
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌflʊəroʊˈjʊərəsɪl/[1] |
| Trade names | Adrucil, Carac, Efudex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682708 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 28 to 100% |
| Protein binding | 8 to 12% |
| Metabolism | Intracellular and liver (CYP-mediated) |
| Elimination half-life | 16 minutes |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C4H3FN2O2 |
| Molar mass | 130.078 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 282–283 °C (540–541 °F) |
| |
| |
| (verify) | |
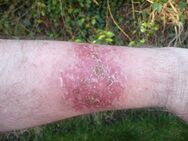
Fluorouracil (5-FU, 5-fluorouracil), sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer.[3] By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer.[3] As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts.[4][5]
Side effects of use by injection are common.[3] They may include inflammation of the mouth, loss of appetite, low blood cell counts, hair loss, and inflammation of the skin.[3] When used as a cream, irritation at the site of application usually occurs.[4] Use of either form in pregnancy may harm the fetus.[3] Fluorouracil is in the antimetabolite and pyrimidine analog families of medications.[6][7] How it works is not entirely clear, but it is believed to involve blocking the action of thymidylate synthase and thus stopping the production of DNA.[3]
Fluorouracil was patented in 1956 and came into medical use in 1962.[8] It is on the World Health Organization's List of Essential Medicines.[9] In 2021, it was the 281st most commonly prescribed medication in the United States, with more than 800,000 prescriptions.[10][11]
Medical uses
Fluorouracil has been given systemically for anal, breast, colorectal, oesophageal, stomach, pancreatic and skin cancers (especially head and neck cancers).[12] It has also been given topically (on the skin) for actinic keratoses, skin cancers and Bowen's disease,[12] and as eye drops for treatment of ocular surface squamous neoplasia.[13] Other uses include ocular injections into a previously created trabeculectomy bleb to inhibit healing and cause scarring of tissue, thus allowing adequate aqueous humor flow to reduce intraocular pressure.
Contraindications
Fluorouracil is contraindicated in patients who are severely debilitated and in patients with bone marrow suppression due to either radiotherapy or chemotherapy.[14] It is likewise contraindicated in pregnant or breastfeeding women.[14] Non-topical use, i.e. administration by injection, should be avoided in patients who do not have malignant illnesses.[14]
Adverse effects
Adverse effects by frequency include:[12][14][15][16][17]
During systemic use
Common (> 1% frequency):
- Nausea
- Vomiting
- Diarrhea (see below for details)
- Mucositis
- Headache
- Hand-foot syndrome
- Myelosuppression (see below for details)
- Alopecia (hair loss)
- Photosensitivity
- Maculopapular eruption
- Itch
- Cardiotoxicity (see below for details)
- Persistent hiccups[18]
- Mood disorders (irritability, anxiety, depression)
Uncommon (0.1–1% frequency):
- Oesophagitis
- GI ulceration and bleeding
- Proctitis
- Nail disorders
- Vein pigmentation
- Confusion
- Cerebellar syndrome
- Encephalopathy
- Visual changes
- Photophobia
- Lacrimation (the expulsion of tears without any emotional or physiologic reason)
Rare (< 0.1% frequency):
- Anaphylaxis
- Allergic reactions
- Fever without signs of infection
- Mania, reversible dementia[19][20]
Diarrhea is severe and may be dose-limiting and is exacerbated by co-treatment with calcium folinate.[12] Neutropenia tends to peak about 9–14 days after beginning treatment.[12] Thrombocytopenia tends to peak about 7–17 days after the beginning of treatment and tends to recover about 10 days after its peak.[12] Cardiotoxicity is a fairly common side effect, usually manifesting as angina or symptoms associated with coronary artery spasm, but about 0.55% of those receiving the drug will develop life-threatening cardiotoxicity.[21] Life-threatening cardiotoxicity includes: arrhythmias, ventricular tachycardia and cardiac arrest, secondary to transmural ischaemia.[21]
During topical use
Common (> 1% frequency):[12][22]
- Local pain
- Itchiness
- Burning
- Stinging
- Crusting
- Weeping
- Dermatitis
- Photosensitivity
Uncommon (0.1–1% frequency):
- Hyper- or hypopigmentation
- Scarring
Neurological damage
The United States package insert warns that acute cerebellar syndrome has been observed following injection of fluorouracil and may persist after cessation of treatment. Symptoms include ataxia, nystagmus, and dysmetria.[23]
Potential overdose
There is very little difference between the minimum effective dose and maximum tolerated dose of 5-FU, and the drug exhibits marked individual pharmacokinetic variability.[24][25][26] Therefore, an identical dose of 5-FU may result in a therapeutic response with acceptable toxicity in some patients and unacceptable and possibly life-threatening toxicity in others.[24] Both overdosing and underdosing are of concern with 5-FU, although several studies have shown that the majority of colorectal cancer patients treated with 5-FU are underdosed based on today's dosing standard, body surface area (BSA).[27][28][29][30] The limitations of BSA-based dosing prevent oncologists from being able to accurately titer the dosage of 5-FU for the majority of individual patients, which results in sub-optimal treatment efficacy or excessive toxicity.[27][28]
Numerous studies have found significant relationships between concentrations of 5-FU in blood plasma and both desirable or undesirable effects on patients.[31][32] Studies have also shown that dosing based on the concentration of 5-FU in plasma can greatly increase desirable outcomes while minimizing negative side effects of 5-FU therapy.[27][33] One such test that has been shown to successfully monitor 5-FU plasma levels and which "may contribute to improved efficacy and safety of commonly used 5-FU-based chemotherapies" is the My5-FU test.[29][34][35]
Interactions
It may increase the INR and prothrombin times in people on warfarin.[14] Fluorouracil's efficacy is decreased when used alongside allopurinol, which can be used to decrease fluorouracil induced stomatitis through use of allopurinol mouthwash.[36]
Pharmacology
Pharmacogenetics
The dihydropyrimidine dehydrogenase (DPD) enzyme is responsible for the detoxifying metabolism of fluoropyrimidines, a class of drugs that includes 5-fluorouracil, capecitabine, and tegafur.[37] Genetic variations within the DPD gene (DPYD) can lead to reduced or absent DPD activity, and individuals who are heterozygous or homozygous for these variations may have partial or complete DPD deficiency; an estimated 0.2% of individuals have complete DPD deficiency.[37][38] Those with partial or complete DPD deficiency have a significantly increased risk of severe or even fatal drug toxicities when treated with fluoropyrimidines; examples of toxicities include myelosuppression, neurotoxicity and hand-foot syndrome.[37][38]
Mechanism of action
5-FU acts in several ways, but principally as a thymidylate synthase (TS) inhibitor. Interrupting the action of this enzyme blocks synthesis of the pyrimidine thymidylate (dTMP), which is a nucleotide required for DNA replication. Thymidylate synthase methylates deoxyuridine monophosphate (dUMP) to form thymidine monophosphate (dTMP). Administration of 5-FU causes a scarcity in dTMP, so rapidly dividing cancerous cells undergo cell death via thymineless death.[39] Calcium folinate provides an exogenous source of reduced folinates and hence stabilises the 5-FU-TS complex, hence enhancing 5-FU's cytotoxicity.[40]
History
In 1954, Abraham Cantarow and Karl Paschkis found liver tumors absorbed radioactive uracil more readily than did normal liver cells. Charles Heidelberger, who had earlier found that fluorine in fluoroacetic acid inhibited a vital enzyme, asked Robert Duschinsky and Robert Schnitzer at Hoffmann-La Roche to synthesize fluorouracil.[41] Some credit Heidelberger and Duschinsky with the discovery that 5-fluorouracil markedly inhibited tumors in mice.[42] The original 1957 report[43][44] In 1958, Anthony R. Curreri, Fred J. Ansfield, Forde A. McIver, Harry A. Waisman, and Charles Heidelberger reported the first clinical findings of 5-FU's activity in cancer in humans.[45]
Natural analogues
In 2003, scientists isolated 5-fluorouracil derivatives, closely related compounds, from the marine sponge, Phakellia fusca, collected around Yongxing Island of the Xisha Islands in the South China Sea. This is significant because fluorine-containing organic compounds are rare in nature, and also because manmade anticancer drugs are not frequently found to have analogues in nature.[46]
Interactive pathway map
Names
The name "fluorouracil" is the INN, USAN, USP name, and BAN. The form "5-fluorouracil" is often used; it shows that there is a fluorine atom on the 5th carbon of a uracil ring.
References
- ↑ "Fluorouracil – Definition and More from the Free Merriam-Webster Dictionary". http://www.merriam-webster.com/dictionary/fluorouracil.
- ↑ "TOLAK : Fluorouracil Cream : 4% (w/w) fluorouracil (as fluorouracil sodium)". https://pdf.hres.ca/dpd_pm/00049486.PDF.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Fluorouracil". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/fluorouracil.html.
- ↑ 4.0 4.1 "Fluorouracil topical". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/fluorouracil-topical.html.
- ↑ "Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders". The Journal of Dermatological Treatment 20 (6): 328–335. 2009. doi:10.3109/09546630902789326. PMID 19954388.
- ↑ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 590. ISBN 9780857111562.
- ↑ (in en) Cancer Chemotherapy: Basic Science to the Clinic. John Wiley & Sons. 2009. p. 76. ISBN 9780470092569. https://books.google.com/books?id=wjjYyYpfiQ8C&pg=PA76.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 511. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA511.
- ↑ The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Fluorouracil - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Fluorouracil.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 978-0-9805790-9-3.
- ↑ "Topical 5-Fluorouracil 1% as Primary Treatment for Ocular Surface Squamous Neoplasia". Ophthalmology 123 (7): 1442–1448. July 2016. doi:10.1016/j.ophtha.2016.02.034. PMID 27030104.
- ↑ 14.0 14.1 14.2 14.3 14.4 "Fluorouracil 50 mg/ml Injection – Summary of Product Characteristics". electronic Medicines Compendium. Hospira UK Ltd. 24 August 2011. http://www.medicines.org.uk/emc/medicine/636/SPC/Fluorouracil++50+mg+ml++Injection/.
- ↑ "DBL Fluorouracil Injection BP" (PDF). TGA eBusiness Services. Hospira Australia Pty Ltd. 21 June 2012. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-02952-3.
- ↑ "ADRUCIL (fluorouracil) injection [Teva Parenteral Medicines, Inc."]. DailyMed. Teva Parenteral Medicines, Inc.. August 2012. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b90e0da7-f702-4f09-9488-74f2bb20e9ac.
- ↑ "Adrucil (fluorouracil) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. http://reference.medscape.com/drug/adrucil-fluorouracil-342092#showall.
- ↑ MedsFacts meta-analysis covering adverse side effect reports of 5fu(fluorouracil) patients who developed hiccups at MedsFact, 2013
- ↑ "Onset of Manic Episode during Chemotherapy with 5-Fluorouracil". Psychiatry Investigation 8 (1): 71–73. March 2011. doi:10.4306/pi.2011.8.1.71. PMID 21519541.
- ↑ Park H. J., Choi Y. T., Kim I. H., Hah J. C.; A case of reversible dementia associated with depression in a patient on 5-FU or its analogue drugs. J. Korean Neuropsychiatr. Assoc. 1987;30:199–202.
- ↑ 21.0 21.1 "Fluorouracil: Martindale: The Complete Drug Reference". London, UK: Pharmaceutical Press. 9 January 2017. https://www.medicinescomplete.com/mc/martindale/current/1836-x.htm.
- ↑ "Efudex, Carac (fluorouracil topical) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. http://reference.medscape.com/drug/efudex-carac-fluorouracil-topical-343545#showall.
- ↑ "Adrucil (Fluorouracil) Injection [TEVA Parenteral Medicines, Inc."]. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=e0794add-67a7-4308-93e9-f889472716cc.
- ↑ 24.0 24.1 "Dose monitoring of 5-fluorouracil in patients with colorectal or head and neck cancer--status of the art". Critical Reviews in Oncology/Hematology 30 (1): 71–79. March 1999. doi:10.1016/s1040-8428(98)00036-5. PMID 10439055.
- ↑ "Dosing strategies for anticancer drugs: the good, the bad and body-surface area". European Journal of Cancer 38 (13): 1677–1684. September 2002. doi:10.1016/s0959-8049(02)00151-x. PMID 12175683.
- ↑ "Role of body surface area in dosing of investigational anticancer agents in adults, 1991-2001". Journal of the National Cancer Institute 94 (24): 1883–1888. December 2002. doi:10.1093/jnci/94.24.1883. PMID 12488482.
- ↑ 27.0 27.1 27.2 "Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study". Clinical Colorectal Cancer 11 (4): 263–267. December 2012. doi:10.1016/j.clcc.2012.05.004. PMID 22683364.
- ↑ 28.0 28.1 "Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens". Clinical Colorectal Cancer 10 (3): 203–206. September 2011. doi:10.1016/j.clcc.2011.03.015. PMID 21855044.
- ↑ 29.0 29.1 "Multicenter evaluation of a novel nanoparticle immunoassay for 5-fluorouracil on the Olympus AU400 analyzer". Therapeutic Drug Monitoring 31 (6): 688–694. December 2009. doi:10.1097/FTD.0b013e3181b9b8c0. PMID 19935361.
- ↑ "The continuum of care: a paradigm for the management of metastatic colorectal cancer". The Oncologist 12 (1): 38–50. January 2007. doi:10.1634/theoncologist.12-1-38. PMID 17227899.
- ↑ "How may anticancer chemotherapy with fluorouracil be individualised?". Clinical Pharmacokinetics 45 (6): 567–592. 2006. doi:10.2165/00003088-200645060-00002. PMID 16719540.
- ↑ "Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic drug monitoring in cancer patients". Pharmacogenomics 14 (7): 799–811. May 2013. doi:10.2217/pgs.13.54. PMID 23651027.
- ↑ "Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer". Journal of Clinical Oncology 26 (13): 2099–2105. May 2008. doi:10.1200/jco.2007.13.3934. PMID 18445839.
- ↑ "Customizing Chemotherapy for Better Cancer Care". MyCare Diagnostics. http://Mycaretests.com.
- ↑ "A Brief History of BSA Dosing". MyCare Diagnostics. http://bettercancercare.com.
- ↑ "Allopurinol mouthwashes in the treatment of 5-fluorouracil-induced stomatitis". American Journal of Clinical Oncology 17 (3): 246–247. June 1994. doi:10.1097/00000421-199406000-00014. PMID 8192112.
- ↑ 37.0 37.1 37.2 "Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing". Clinical Pharmacology and Therapeutics 94 (6): 640–645. December 2013. doi:10.1038/clpt.2013.172. PMID 23988873.
- ↑ 38.0 38.1 "Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity". Pharmacogenomics 12 (9): 1321–1336. September 2011. doi:10.2217/pgs.11.72. PMID 21919607.
- ↑ "5-fluorouracil: mechanisms of action and clinical strategies". Nature Reviews. Cancer 3 (5): 330–338. May 2003. doi:10.1038/nrc1074. PMID 12724731.
- ↑ "5-Fluorouracil derivatives: a patent review". Expert Opinion on Therapeutic Patents 22 (2): 107–123. February 2012. doi:10.1517/13543776.2012.661413. PMID 22329541.
- ↑ Drug discovery: a history.. John Wiley & Sons. June 2005. p. 255.
- ↑ "50 years ago in cell biology: A virologist recalls his work on cell growth inhibition". The Scientist. 30 January 2008. http://www.the-scientist.com/news/display/54259/.
- ↑ "Ode to 5-Fluorouracil". Clinical Colorectal Cancer 6 (9): 609. September 2007. doi:10.3816/CCC.2007.n.029. http://cigjournals.metapress.com/content/b464v2u31594jj28/.
- ↑ "Fluorinated pyrimidines, a new class of tumour-inhibitory compounds". Nature 179 (4561): 663–666. March 1957. doi:10.1038/179663a0. PMID 13418758. Bibcode: 1957Natur.179..663H.
- ↑ "A Retrospective: On Clinical Studies with 5-Fluorouracil". Cancer Research (American Association for Cancer Research) 76 (4): 767–768. February 2016. doi:10.1158/0008-5472.CAN-16-0150. PMID 26880809. https://cancerres.aacrjournals.org/content/76/4/767. Retrieved 17 August 2019.
- ↑ "5-Fluorouracil derivatives from the sponge Phakellia fusca". Journal of Natural Products 66 (2): 285–288. February 2003. doi:10.1021/np020034f. PMID 12608868.
Further reading
- "Fluorouracil Therapy and DPYD Genotype". Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). 2016. Bookshelf ID: NBK395610. https://www.ncbi.nlm.nih.gov/books/NBK395610/.
- "5-Fluorouracil toxicity and dihydropyrimidine dehydrogenase enzyme: implications for practice". Clinical Journal of Oncology Nursing 18 (5): 581–585. October 2014. doi:10.1188/14.CJON.581-585. PMID 25253112.
External links
 |