Chemistry:Glutaraldehyde
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentanedial[1] | |
| Other names
Glutaraldehyde
Glutardialdehyde Glutaric acid dialdehyde Glutaric aldehyde Glutaric dialdehyde 1,5-Pentanedial | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H8O2 | |
| Molar mass | 100.117 |
| Appearance | Clear liquid |
| Odor | pungent[2] |
| Density | 1.06 g/mL |
| Melting point | −14 °C (7 °F; 259 K) |
| Boiling point | 187 °C (369 °F; 460 K) |
| Miscible, reacts | |
| Vapor pressure | 17 mmHg (20°C)[2] |
| Hazards | |
| Safety data sheet | CAS 111-30-8 |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H302, H314, H317, H331, H334, H400 | |
| P260, P264, P270, P271, P272, P273, P280, P284, P301+312, P330, P302+352, P332+313, P304+340, P305+351+338, P311, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | noncombustible[2] |
Threshold limit value (TLV)
|
0.2 ppm (0.82 mg/m3) (TWA), 0.05 ppm (STEL) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
134 mg/kg (rat, oral); 2,560 mg/kg (rabbit, dermal) |
| NIOSH (US health exposure limits): | |
REL (Recommended)
|
0.2 ppm (0.8 mg/m3)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glutaraldehyde is an organic compound with the formula (CH
2)
3(CHO)
2. The molecule consists of a five carbon chain doubly terminated with formyl (CHO) groups. It is usually used as a solution in water, and such solutions exists as a collection of hydrates, cyclic derivatives, and condensation products, several of which interconvert. Because the molecule has two carbonyl group that are reactive to primary amine groups (even as its hydrates), it can function as a crosslinking agent for any substance with primary amine groups and develop imine connected links. Crosslinking rigidifies and deactivates many biological functions, so in this way, glutaraldehyde solutions are used as biocides and as fixative. It is sold under the brandname Cidex and Glutaral.[3][4][5][6] As a disinfectant, it is used to sterilize surgical instruments.[3]
Uses
Biochemistry
Glutaraldehyde is used in biochemistry applications as an amine-reactive homobifunctional crosslinker and fixative.[7][8] It kills cells quickly by crosslinking their proteins. It is usually employed alone or mixed with formaldehyde[9] as the first of two fixative processes to stabilize specimens such as bacteria, plant material, and human cells. A second fixative procedure uses osmium tetroxide to crosslink and stabilize cell and organelle membrane lipids.
Another application for treatment of proteins with glutaraldehyde is the inactivation of bacterial toxins to generate toxoid vaccines, e.g., the pertussis (whooping cough) toxoid component in the Boostrix Tdap vaccine produced by GlaxoSmithKline.[10]
Material Science
In material science glutaraldehyde application areas range from polymers to metals and biomaterials. Glutaraldehyde is commonly used as fixing agent before characterization of biomaterials for microscopy. Glutaraldehyde is a powerful crosslinking agent for many polymers containing primary amine groups.[citation needed]. Glutaraldehdye also can be used for an interlinking agent to improve the adhesion force between two polymeric coatings.[11] Glutaraldehyde is also used to protect against corrosion of undersea pipes.[12]
Medical
Clinical uses
Glutaraldehyde is used as a disinfectant and medication.[3][4][13] Usually applied as a solution, it is used to sterilize surgical instruments and other areas.[3]
Dermatological uses
As a medication it is used to treat plantar warts.[4] For this purpose, a 10% w/v solution is used. It dries the skin, facilitating physical removal of the wart.[14]
Glutaraldehyde is also used in the treatment of hyperhidrosis under the control of dermatologists. In people who have frequent sweating but do not respond to aluminum chloride. Glutaraldehyde solution is an effective agent to treat palmar and plantar hyperhidrosis as an alternative to tannic acid and formaldehyde.[15]
Other Uses
Use in the Aquarium Hobby
Glutaraldehyde diluted with water is often marketed as alternative to carbon dioxide gas injection for aquarium plants. It is commonly also used by aquarists in low doses as an algaecide.[16]
Safety
Side effects include skin irritation.[4] If exposed to large amounts, nausea, headache, and shortness of breath may occur.[3] Protective equipment is recommended when used, especially in high concentrations.[3] Glutaraldehyde is effective against a range of microorganisms including spores.[3][17] Glutaraldehyde is a dialdehyde.[18] It works by a number of mechanisms.[17]
As a strong sterilant, glutaraldehyde is toxic and a strong irritant.[19] There is no strong evidence of carcinogenic activity,[20] However, some occupations that work with this chemical have an increased risk of some cancers.[20]
Production and reactions
Production
Glutaraldehyde is produced industrially by the catalytic oxidation of cyclopentene by hydrogen peroxide, which can be achieved in the presence of various tungstic acid-based heteropoly acid catalysts.[21][22] This reaction essentially mimics ozonolysis. Alternatively it can be made by the Diels-Alder reaction of acrolein and vinyl ethers followed by hydrolysis.[23]
Reactions
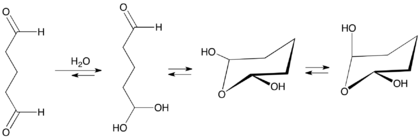
Like other dialdehydes, (e.g., glyoxal) and simple aldehydes (e.g., formaldehyde), glutaraldehyde hydrates in aqueous solution, forming gem-diols. These diols in turn equilibrate with cyclic hemiacetal.[24][23][7] Monomeric glutaraldehyde polymerizes by aldol condensation and Michael reactions yielding alpha, beta-unsaturated poly-glutaraldehyde and related oligomers. This reaction occurs at alkaline pH values.[25]
A number of mechanisms have been invoked to explain the biocidal and fixative properties of glutaraldehyde.[17] Like many other aldehydes, it reacts with primary amines and thiol groups, which are common functional groups in proteins, nucleic acids and polymeric materials. Being bi-functional, glutaraldehyde is a crosslinker, which rigidifies macromolecular structures and shuts down their reactivity.[26]

The aldehyde groups in glutaraldehyde are susceptible to formation of imines by reaction with the amines of lysine and nucleic acids. The derivatives from aldol condensation of pairs of glutaraldehyde also undergo imine formation.[25]
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 907. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 2.3 "CDC - NIOSH Pocket Guide to Chemical Hazards -Glutaraldehyde". https://www.cdc.gov/niosh/npg/npgd0301.html.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 WHO Model Formulary 2008. World Health Organization. 2009. pp. 323, 325. ISBN 9789241547659.
- ↑ 4.0 4.1 4.2 4.3 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 825. ISBN 9780857111562.
- ↑ Bonewit-West, Kathy (2015) (in en). Clinical Procedures for Medical Assistants. Elsevier Health Sciences. p. 96. ISBN 9781455776610. https://books.google.com/books?id=pTwcBgAAQBAJ&pg=PA96. Retrieved 9 September 2017.
- ↑ Sullivan, John Burke; Krieger, Gary R. (2001) (in en). Clinical Environmental Health and Toxic Exposures. Lippincott Williams & Wilkins. p. 601. ISBN 9780683080278. https://books.google.com/books?id=PyUSgdZUGr4C&pg=PA601. Retrieved 19 September 2020.
- ↑ 7.0 7.1 Srinivasan, Mythily; Sedmak, Daniel; Jewell, Scott (2002). "Effect of Fixatives and Tissue Processing on the Content and Integrity of Nucleic Acids". The American Journal of Pathology 161 (6): 1961–1971. doi:10.1016/S0002-9440(10)64472-0. PMID 12466110.
- ↑ Vakili, Mohammadtaghi; Rafatullah, Mohd; Salamatinia, Babak; Abdullah, Ahmad Zuhairi; Ibrahim, Mahamad Hakimi; Tan, Kok Bing; Gholami, Zahra; Amouzgar, Parisa (2014). "Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review". Carbohydrate Polymers 113: 115–130. doi:10.1016/j.carbpol.2014.07.007. PMID 25256466.
- ↑ Karnovsky, M.J. (1965). A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology 27: 137A–138A
- ↑ Boostrix prescribing information , ©2009, GlaxoSmithKline
- ↑ Erisen, Deniz. E (23 September 2022), "A novel chitosan and polydopamine interlinked bioactive coating for metallic biomaterials", Journal of Materials Science: Materials in Medicine (Springer-Nature) 33 (10): 65, doi:10.1007/s10856-022-06688-x, ISSN 1573-4838, PMID 36138240
- ↑ Falck, Christian; Kleppe, Terje; Maribu, Jarleiv (1993-12-23). Commissioning of long subsea pipelines - environmental aspects. Norway (published 1997-12-31). https://inis.iaea.org/collection/NCLCollectionStore/_Public/29/045/29045754.pdf.
- ↑ Bonewit-West, Kathy (2015) (in en). Clinical Procedures for Medical Assistants. Elsevier Health Sciences. p. 96. ISBN 9781455776610. https://books.google.com/books?id=pTwcBgAAQBAJ&pg=PA96.
- ↑ NHS Choices: Glutarol
- ↑ Juhlin, L; Hansson, H (1968), "Topical glutaraldehyde for plantar hyperhidrosis", Archives of Dermatology (American Medical Association) 97 (3): 327–330, doi:10.1001/archderm.1968.01610090099017, ISSN 0003-987X, PMID 5641337, https://jamanetwork.com/journals/jamadermatology/article-abstract/530562
- ↑ Antiquis, Avus (20 September 2017). "Glutaraldehyde Revisited". https://praquatics.com/forums/threads/glutaraldehyde-revisited.2566/.
- ↑ 17.0 17.1 17.2 Fraise, Adam P.; Maillard, Jean-Yves; Sattar, Syed (2012) (in en). Russell, Hugo and Ayliffe's Principles and Practice of Disinfection, Preservation and Sterilization. John Wiley & Sons. p. Chapter 2. ISBN 9781118425862. https://books.google.com/books?id=b6Pz6kMw3akC&pg=PT50.
- ↑ Pfafflin, James R.; Ziegler, Edward N. (2006) (in en). Encyclopedia of Environmental Science and Engineering: A-L. CRC Press. p. 235. ISBN 9780849398438. https://books.google.com/books?id=ylktfmkJQZwC&pg=PA235. Retrieved 19 September 2020.
- ↑ Canadian Centre for Occupational Health and Safety (CCOHS) (a federal government site) > OSH Answers > Diseases, Disorders & Injuries > Asthma Document last updated on 8 February 2005
- ↑ 20.0 20.1 Toxicology and Carcinogenesis Studies of Glutaraldehyde
- ↑ Chandler, Malcolm (15 April 2001). "Hydrogen Peroxide-Tungstic Acid". Encyclopedia of Reagents for Organic Synthesis: rh046. doi:10.1002/047084289X.rh046. ISBN 0471936235.
- ↑ Furukawa, Hiroshi; Nakamura, Teiji; Inagaki, Hiroyuki; Nishikawa, Eiichiro; Imai, Chihiro; Misono, Makoto (5 May 1988). "Oxidation of Cyclopentene with Hydrogen Peroxide Catalyzed by 12-Heteropoly Acids". Chemistry Letters 17 (5): 877–880. doi:10.1246/cl.1988.877. https://www.journal.csj.jp/doi/pdf/10.1246/cl.1988.877.
- ↑ 23.0 23.1 Christian Kohlpaintner; Markus Schulte; Jürgen Falbe; Peter Lappe; Jürgen Weber (2008). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub2.
- ↑ Whipple Earl B.; Ruta Michael (1974). "Structure of Aqueous Glutaraldehyde". J. Org. Chem. 39 (12): 1666–1668. doi:10.1021/jo00925a015.
- ↑ 25.0 25.1 Migneault, Isabelle; Dartiguenave, Catherine; Bertrand, Michel J.; Waldron, Karen C. (2004). "Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking". BioTechniques 37 (5): 790–802. doi:10.2144/04375RV01. PMID 15560135.
- ↑ H. Uhr; B. Mielke; O. Exner; K. R. Payne; E. Hill (2013). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_563.pub2.
External links
- "Glutaraldehyde". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/glutaraldehyde.
- Glutaraldehyde: Sources of emissions AU National Pollutant Inventory
- Glutaraldehyde US National Institute for Occupational Safety and Health
- Glutaraldehyde NIST Standard Reference Data
 |