Chemistry:Hexacene
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexacene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C26H16 | |
| Molar mass | 328.41 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
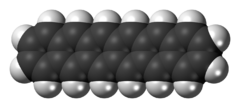
Hexacene is an aromatic compound consisting of six linearly-fused benzene rings. It is a blue-green, air-stable solid with low solubility.[1]
Hexacene is one of a series of linear polycyclic molecules created by such aromatic ring fusions, a series termed acenes—the previous in the series being pentacene (with five fused rings) and the next being heptacene (with seven). It and other acenes and their derivatives have been investigated in potential applications related to organic semiconductors. Like larger acenes, hexacene is poorly soluble, but derivatives have been prepared with improved solubility. 6,15-Bis(tri-t-butylsilylethynyl)hexacene, which melts with decomposition at 96 °C.[2]
Syntheses and structure
Hexacene has been the subject of many syntheses. One route entails by thermal decarbonylation of monoketone precursor.[1]
Further reading
- First synthesis:
- Marschalk, C. Linear hexacenes. Bull. Soc. Chim. Fr. 6, 1112–1121 (1939).
- E. Clar (1939). "Hexacen, ein grüner, einfacher Kohlenwasserstoff (Aromatische Kohlenwasserstoffe, XXIV. Mitteil) (trans=Hexacene, a Green Simple Hydrocarbon (Aromatic hydrocarbons. XXIV.)". Ber. Dtsch. Chem. Ges. B 72B: 1817–1821. doi:10.1002/cber.19390721002.
- E. Clar (1942). "Eine neue Synthese des Hexacens (Aromatische Kohlenwasserstoffe, XXXIV. Mitteil)". Berichte der deutschen chemischen Gesellschaft 75 (11): 1283–1287. doi:10.1002/cber.19420751102.
- By dehydrogenation of hexacosahydrohexacene by palladium on carbonBailey, W.J.; Liao, C.W. (1955). "Cyclic Dienes. XI. New Syntheses of Hexacene and Heptacene". J. Am. Chem. Soc. 77 (4): 992–993. doi:10.1021/ja01609a055.
- Isolation: Angliker H.; Rommel E.; Wirz J. (1982). "Electronic spectra of hexacene in solution (ground state, triplet state, dication and dianion)". Chemical Physics Letters 87 (2): 208–12. doi:10.1016/0009-2614(82)83589-6. Bibcode: 1982CPL....87..208A.
- By decarbonylation of a diketone precursor:Mondal, R.; Adhikari, R.M.; Shah, B.K.; Neckers, D.C. (2007). "Revisiting the Stability of Hexacenes". Org. Lett. 9 (13): 2505–2508. doi:10.1021/ol0709376. PMID 17516652.
- Deoxygenation route: Krüger, Justus; Eisenhut, Frank; Alonso, José M.; Lehmann, Thomas; Guitián, Enrique; Pérez, Dolores; Skidin, Dmitry; Gamaleja, Florian et al. (2017). "Imaging the electronic structure of on-surface generated hexacene". Chem. Commun. 53 (10): 1583–1586. doi:10.1039/C6CC09327B. ISSN 1359-7345. PMID 27990553.
External links
- Molecular graphics representation of the X-ray crystallographic structure determined for 6,15-bis(tri-t-butylsilylethynyl)hexacene—hexacene with bulky, protective substituents in the reactive 6- and 15- ring positions, see rotating graphic at base of page.
- Sander-Wise Polycyclic Aromatic Hydrocarbon Structure Index entry for hexacene, NIST Special Publication 922.
References
- ↑ 1.0 1.1 1.2 Watanabe, M.; Chang, Y.J.; Liu, S.-W.; Chao, T.-H.; Goto, K.; Islam, M.M.; Yuan, C.H.; Tao, Y.T. et al. (2012). "The synthesis, crystal structure and charge-transport properties of hexacene". Nature Chemistry 4 (7): 574–578. doi:10.1038/nchem.1381. PMID 22717444. Bibcode: 2012NatCh...4..574W.
- ↑ Payne M. M.; Parkin S. R.; Anthony J. E. (2005). "Functionalized higher acenes: hexacene and heptacene". Journal of the American Chemical Society 127 (22): 8028–9. doi:10.1021/ja051798v. PMID 15926823.
 |

