Biology:Btk-type zinc finger
| Btk motif | |||||||||
|---|---|---|---|---|---|---|---|---|---|
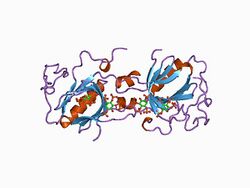 ph domain and btk motif from bruton's tyrosine kinase mutant e41k in complex with ins(1,3,4,5)p4 | |||||||||
| Identifiers | |||||||||
| Symbol | BTK | ||||||||
| Pfam | PF00779 | ||||||||
| InterPro | IPR001562 | ||||||||
| SMART | BTK | ||||||||
| SCOP2 | 1btk / SCOPe / SUPFAM | ||||||||
| |||||||||
In molecular biology, the Btk-type zinc finger or Btk motif (BM) is a conserved zinc-binding motif containing conserved cysteines and a histidine that is present in certain eukaryotic signalling proteins. The motif is named after Bruton's tyrosine kinase (Btk), an enzyme which is essential for B cell maturation in humans and mice.[1][2] Btk is a member of the Tec family of protein tyrosine kinases (PTK). These kinases contain a conserved Tec homology (TH) domain between the N-terminal pleckstrin homology (PH) domain and the Src homology 3 (SH3) domain. The N-terminal of the TH domain is highly conserved and known as the Btf motif, while the C-terminal region of the TH domain contains a proline-rich region (PRR). The Btk motif contains a conserved His and three Cys residues that form a zinc finger (although these differ from known zinc finger topologies), while PRRs are commonly involved in protein-protein interactions, including interactions with G proteins.[3][4] The TH domain may be of functional importance in various signalling pathways in different species.[1] A complete TH domain, containing both the Btk and PRR regions, has not been found outside the Tec family; however, the Btk motif on its own does occur in other proteins, usually C-terminal to a PH domain (note that although a Btk motif always occurs C-terminal to a PH domain, not all PH domains are followed by a Btk motif).
The crystal structures of Btk show that the Btk-type zinc finger has a globular core, formed by a long loop which is held together by a zinc ion, and that the Btk motif is packed against the PH domain.[1] The zinc-binding residues are a histidine and three cysteines, which are fully conserved in the Btk motif.[5]
Proteins known to contain a Btk-type zinc finger include:
- Mammalian Bruton's tyrosine kinase (Btk), a protein tyrosine kinase involved in modulation of diverse cellular processes. Mutations affecting Btk are the cause of X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency in mice.
- Mammalian Tec, Bmx, and Itk proteins, which are tyrosine protein kinases of the Tec subfamily.
- Drosophila tyrosine-protein kinase Btk29A, which is required for the development of proper ring canals and of male genitalia and required for adult survival.
- Mammalian Ras GTPase-activating proteins (RasGAP), which regulate the activation of inactive GDP-bound Ras by converting GDP to GTP.
References
- ↑ 1.0 1.1 1.2 "Tec homology (TH) adjacent to the PH domain". FEBS Lett. 350 (2–3): 263–5. August 1994. doi:10.1016/0014-5793(94)00783-7. PMID 8070576.
- ↑ "Bruton's tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling". Immunol. Rev. 203: 200–15. February 2005. doi:10.1111/j.0105-2896.2005.00225.x. PMID 15661031.
- ↑ "Missense mutations affecting a conserved cysteine pair in the TH domain of Btk". FEBS Lett. 413 (2): 205–10. August 1997. doi:10.1016/S0014-5793(97)00912-5. PMID 9280283.
- ↑ "The G protein G alpha12 stimulates Bruton's tyrosine kinase and a rasGAP through a conserved PH/BM domain". Nature 395 (6704): 808–13. October 1998. doi:10.1038/27454. PMID 9796816. Bibcode: 1998Natur.395..808J.
- ↑ "Structure of the PH domain and Btk motif from Bruton's tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia". EMBO J. 16 (12): 3396–404. June 1997. doi:10.1093/emboj/16.12.3396. PMID 9218782.
 |

