Medicine:Hypervitaminosis A
| Hypervitaminosis A | |
|---|---|
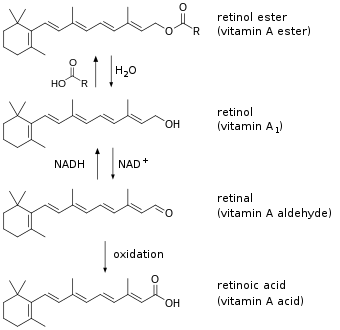 | |
| Forms of preformed vitamin A in the body | |
| Specialty | Toxicology |
Hypervitaminosis A refers to the toxic effects of ingesting too much preformed vitamin A (retinyl esters, retinol, and retinal). Symptoms arise as a result of altered bone metabolism and altered metabolism of other fat-soluble vitamins. Hypervitaminosis A is believed to have occurred in early humans, and the problem has persisted throughout human history. Toxicity results from ingesting too much preformed vitamin A from foods (such as fish liver or animal liver), supplements, or prescription medications and can be prevented by ingesting no more than the recommended daily amount.
Diagnosis can be difficult, as serum retinol is not sensitive to toxic levels of vitamin A, but there are effective tests available. Hypervitaminosis A is usually treated by stopping intake of the offending food(s), supplement(s), or medication. Most people make a full recovery. High intake of provitamin carotenoids (such as beta-carotene) from vegetables and fruits does not cause hypervitaminosis A.
Signs and symptoms
Symptoms may include:
- Changes in consciousness
- Decreased appetite
- Dizziness[1]
- Vision changes, double vision (in young children)
- Drowsiness
- Headache
- Irritability[1]
- Nausea[1]
- Vomiting[1]
Signs
- Poor weight gain (in infants and children)
- Skin and hair changes
- Cracking at corners of the mouth
- Hair loss (alopecia)
- Higher sensitivity to sunlight
- Swelling of lips (cheilitis)
- Dryness of lips, mouth, eyes, and inside the nose[1]
- Skin peeling, itching
- Yellow discoloration of the skin (aurantiasis cutis)
- Abnormal softening of the skull bone (craniotabes in infants and children)
- Blurred vision[1]
- Bone pain or swelling
- Bulging fontanelle (in infants)
- Gastric mucosal calcinosis[2]
- Heart valve calcification[3]
- Hypercalcemia
- Increased intracranial pressure manifesting as cerebral edema, papilledema, and headache (may be referred to as idiopathic intracranial hypertension)[4][5]
- Liver damage[6][7][8][9][10][11][12][13][14]
- Premature epiphyseal closure[15][16][17][18][19]
- Spontaneous fracture[20][21]
- Uremic pruritus[22]
Causes
Hypervitaminosis A results from excessive intake of preformed vitamin A. Genetic variations in tolerance to vitamin A intake may occur, so the toxic dose will not be the same for everyone.[23] Children are particularly sensitive to vitamin A, with daily intakes of 1500 IU/kg body weight reportedly leading to toxicity.[21]
Types of vitamin A
- It is "largely impossible" for provitamin carotenoids, such as beta-carotene, to cause toxicity, as their conversion to retinol is highly regulated.[21] No vitamin A toxicity has ever been reported from ingestion of excessive amounts.[24] Overconsumption of beta-carotene can only cause carotenosis, a harmless and reversible cosmetic condition in which the skin turns orange.
- Preformed vitamin A absorption and storage in the liver occur very efficiently until a pathologic condition develops.[21] When ingested, 70–90% of preformed vitamin A is absorbed and used.[21]
Sources of toxicity
- Diet – Liver is high in vitamin A. The liver of certain animals, including the polar bear, bearded seal,[25][26] fish,[27] walrus,[28] and moose,[29] are particularly toxic (see Liver (food) § Poisoning). It has been estimated that consumption of 500 grams of polar bear liver would result in a toxic dose for a human.[25]
- Supplements – Dietary supplements can be toxic when taken above recommended dosages.[citation needed]
Types of toxicity
- Acute toxicity occurs over a period of hours or a few days, and is less of a problem than chronic toxicity.
- Chronic toxicity results from daily intakes greater than 25,000 IU for 6 years or longer and more than 100,000 IU for 6 months or longer.
Mechanism
Retinol is absorbed and stored in the liver very efficiently until a pathologic condition develops.[21]
Delivery to tissues
Absorption
When ingested, 70–90% of preformed vitamin A is absorbed and used.[21]
According to a 2003 review, water-miscible, emulsified, and solid forms of vitamin A supplements are more toxic than oil-based supplement and liver sources.[30]
Storage
80–90% of the total body reserves of preformed vitamin A are in the liver (with 80–90% of this amount being stored in hepatic stellate cells and the remaining 10–20% being stored in hepatocytes). Fat is another significant storage site, while the lungs and kidneys may also be capable of storage.[21]
Transport
Until recently, it was thought that the sole important retinoid delivery pathway to tissues involved retinol bound to retinol-binding protein (RBP4). More recent findings, however, indicate that retinoids can be delivered to tissues through multiple overlapping delivery pathways, involving chylomicrons, very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL), retinoic acid bound to albumin, water-soluble β-glucuronides of retinol and retinoic acid, and provitamin A carotenoids.[31]
The range of serum retinol concentrations under normal conditions is 1–3 μmol/L. Elevated amounts of retinyl ester (i.e., >10% of total circulating vitamin A) in the fasting state have been used as markers for chronic hypervitaminosis A in humans. Candidate mechanisms for this increase include decreased hepatic uptake of vitamin A and the leaking of esters into the bloodstream from saturated hepatic stellate cells.[21]
Effects
Effects include increased bone turnover and altered metabolism of fat-soluble vitamins. More research is needed to fully elucidate the effects.
Increased bone turnover
Retinoic acid suppresses osteoblast activity and stimulates osteoclast formation in vitro,[24] resulting in increased bone resorption and decreased bone formation. It is likely to exert this effect by binding to specific nuclear receptors (members of the retinoic acid receptor or retinoid X receptor nuclear transcription family) which are found in every cell (including osteoblasts and osteoclasts).[citation needed]
This change in bone turnover is likely to be the reason for numerous effects seen in hypervitaminosis A, such as hypercalcemia and numerous bone changes such as bone loss that potentially leads to osteoporosis, spontaneous bone fractures, altered skeletal development in children, skeletal pain, radiographic changes,[21][24] and bone lesions.[32]
Altered fat-soluble vitamin metabolism
Preformed vitamin A is fat-soluble and high levels have been reported to affect metabolism of the other fat-soluble vitamins D,[24] E, and K.
The toxic effects of preformed vitamin A might be related to altered vitamin D metabolism, concurrent ingestion of substantial amounts of vitamin D, or binding of vitamin A to receptor heterodimers. Antagonistic and synergistic interactions between these two vitamins have been reported, as they relate to skeletal health.
Stimulation of bone resorption by vitamin A has been reported to be independent of its effects on vitamin D.[24]
Mitochondrial toxicity
Preformed vitamin A and retinoids exerts several toxic effects regarding redox environment and mitochondrial function. [33]
Diagnosis
Retinol concentrations are nonsensitive indicators
Assessing vitamin A status in persons with subtoxicity or toxicity is complicated because serum retinol concentrations are not sensitive indicators in this range of liver vitamin A reserves.[21] The range of serum retinol concentrations under normal conditions is 1–3 μmol/L and, because of homeostatic regulation, that range varies little with widely disparate vitamin A intakes.[21]
Retinol esters have been used as markers
Retinyl esters can be distinguished from retinol in serum and other tissues and quantified with the use of methods such as high-performance liquid chromatography.[21]
Elevated amounts of retinyl ester (i.e., >10% of total circulating vitamin A) in the fasting state have been used as markers for chronic hypervitaminosis A in humans and monkeys.[21] This increased retinyl ester may be due to decreased hepatic uptake of vitamin A and the leaking of esters into the bloodstream from saturated hepatic stellate cells.[21]
Prevention
Hypervitaminosis A can be prevented by not ingesting more than the US Institute of Medicine Daily Tolerable Upper Level of intake for Vitamin A. This level is for synthetic and natural retinol ester forms of vitamin A. Carotene forms from dietary sources are not toxic. Possible pregnancy, liver disease, high alcohol consumption, and smoking are indications for close monitoring and limitation of vitamin A administration.[citation needed]
Daily tolerable upper level
| Life stage group category | Upper Level (μg/day) |
|---|---|
| Infants
0–6 months |
600 600 |
| Children and adolescents
1–3 years |
600 900 1700 2800 |
| Adults
19 – 70 years |
3000 |
Treatment
- Stopping high vitamin A intake is the standard treatment. Most people fully recover.
- Phosphatidylcholine (in the form of PPC or DLPC), the substrate for lecithin retinol acyltransferase, which converts retinol into retinyl esters (the storage forms of vitamin A).
- Vitamin E may alleviate hypervitaminosis A.[34]
- Liver transplantation may be a valid option if no improvement occurs.[35]
If liver damage has progressed into fibrosis, synthesizing capacity is compromised and supplementation can replenish PC. However, recovery is dependent on removing the causative agent: halting high vitamin A intake.[36][37][38][39]
History
Vitamin A toxicity is known to be an ancient phenomenon; fossilized skeletal remains of early humans suggest bone abnormalities may have been caused by hypervitaminosis A.[21] There are two theories for hypervitaminosis A in the isolated case of KMN-ER 1808. One of these is the increased consumption of meat and the second is an increase in insect consumption, or entomophagy.[citation needed]
Vitamin A toxicity has long been known to the Inuit as they will not eat the liver of polar bears or bearded seals due to them containing dangerous amounts of Vitamin A.[25] It has been known to Europeans since at least 1597 when Gerrit de Veer wrote in his diary that, while taking refuge in the winter in Nova Zemlya, he and his men became severely ill after eating polar bear liver.[40]
In 1913, Antarctic explorers Douglas Mawson and Xavier Mertz were both poisoned (and Mertz died) from eating the livers of their sled dogs during the Far Eastern Party.[41] Another study suggests, however, that exhaustion and diet change are more likely to have caused the tragedy.[42]
Other animals
Some Arctic animals demonstrate no signs of hypervitaminosis A despite having 10–20 times the level of vitamin A in their livers as other Arctic animals. These animals are top predators and include the polar bear, Arctic fox, bearded seal, and glaucous gull. This ability to efficiently store higher amounts of vitamin A may have contributed to their survival in the extreme environment of the Arctic.[43]
Treatment
These treatments have been used to help treat or manage toxicity in animals. Although not considered part of standard treatment, they might be of some benefit to humans.
- Vitamin E appears to be an effective treatment in rabbits,[44] and prevents side effects in chicks[45]
- Taurine significantly reduces toxic effects in rats.[46] Retinoids can be conjugated by taurine and other substances. Significant amounts of retinotaurine are excreted in the bile,[47] and this retinol conjugate is thought to be an excretory form, as it has little biological activity.[48]
- Red yeast rice ("cholestin") – significantly reduces toxic effects in rats.[49]
- Vitamin K prevents hypoprothrombinemia in rats and can sometimes control the increase in plasma/cell ratios of vitamin A.[50]
See also
- Vitamin poisoning
- Far Eastern Party
- Retinoic acid syndrome
- Piblokto
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Olson, Jazmine M.; Ameer, Muhammad Atif; Goyal, Amandeep (2023), "Vitamin A Toxicity", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 30422511, https://www.ncbi.nlm.nih.gov/books/NBK532916/, retrieved 2023-12-18
- ↑ "Gastric mucosal calcinosis: clinicopathologic considerations". Advances in Anatomic Pathology 14 (3): 224–8. May 2007. doi:10.1097/PAP.0b013e31805048ea. PMID 17452819. https://zenodo.org/record/1234899.
- ↑ "Increased dietary intake of vitamin A promotes aortic valve calcification in vivo". Arteriosclerosis, Thrombosis, and Vascular Biology 33 (2): 285–93. February 2013. doi:10.1161/ATVBAHA.112.300388. PMID 23202364.
- ↑ Libien, J.; Kupersmith, M. J.; Blaner, W.; McDermott, M. P.; Gao, S.; Liu, Y.; Corbett, J.; Wall, M. et al. (2017-01-15). "Role of vitamin A metabolism in IIH: Results from the idiopathic intracranial hypertension treatment trial". Journal of the Neurological Sciences 372: 78–84. doi:10.1016/j.jns.2016.11.014. ISSN 1878-5883. PMID 28017254.
- ↑ "Idiopathic intracranial hypertension (pseudotumor cerebri)". Current Neurology and Neuroscience Reports 8 (2): 87–93. March 2008. doi:10.1007/s11910-008-0015-0. PMID 18460275. https://link.springer.com/10.1007/s11910-008-0015-0.
- ↑ "Vitamin A toxicity in a physical culturist patient: a case report and review of the literature". Annals of Hepatology 5 (4): 293–395. 2006. doi:10.1016/S1665-2681(19)31992-1. PMID 17151585.
- ↑ "Vitamin A hepatotoxicity in multiple family members". Hepatology 8 (2): 272–5. 1988. doi:10.1002/hep.1840080214. PMID 3356407.
- ↑ "Stellate-cell lipidosis in liver biopsy specimens. Recognition and significance". American Journal of Clinical Pathology 119 (2): 254–8. February 2003. doi:10.1309/6DKC-03C4-GAAE-N2DK. PMID 12579996.
- ↑ "[Cirrhosis of the liver and esophageal bleeding after chronic vitamin A intoxication (author's transl)]". Leber, Magen, Darm 10 (4): 193–7. August 1980. PMID 6969836.
- ↑ "Vitamin A abuse: development of cirrhosis despite cessation of vitamin A. A six-year clinical and histopathologic follow-up". Liver 12 (6): 381–6. December 1992. doi:10.1111/j.1600-0676.1992.tb00592.x. PMID 1470008.
- ↑ "Cirrhosis due to hypervitaminosis A". The Western Journal of Medicine 128 (3): 244–6. March 1978. PMID 636413.
- ↑ "Possible role of endogenous retinoid (Vitamin A) toxicity in the pathophysiology of primary biliary cirrhosis". Journal of Theoretical Biology 206 (1): 47–54. September 2000. doi:10.1006/jtbi.2000.2102. PMID 10968936. Bibcode: 2000JThBi.206...47E.
- ↑ "Fatty liver in hypervitaminosis A: synthesis and release of hepatic triglycerides". The American Journal of Physiology 234 (5): E511–4. May 1978. doi:10.1152/ajpendo.1978.234.5.E511. PMID 645903. http://ajpendo.physiology.org/cgi/pmidlookup?view=reprint&pmid=645903.
- ↑ "Hypervitaminosis A-induced liver fibrosis: stellate cell activation and daily dose consumption". Liver International 26 (2): 182–6. March 2006. doi:10.1111/j.1478-3231.2005.01207.x. PMID 16448456.
- ↑ "Hypervitaminosis A in the dog". American Journal of Veterinary Research 36 (11): 1597–1603. November 1975. PMID 1190603.
- ↑ "Local disappearance of epiphyseal growth plates in rats with hypervitaminosis A". The Journal of Veterinary Medical Science 60 (7): 815–21. July 1998. doi:10.1292/jvms.60.815. PMID 9713809.
- ↑ "Immunohistochemical observations on the initial disorders of the epiphyseal growth plate in rats induced by high dose of vitamin A". The Journal of Veterinary Medical Science 61 (3): 233–8. March 1999. doi:10.1292/jvms.61.233. PMID 10331194.
- ↑ "Histological disorders related to the focal disappearance of the epiphyseal growth plate in rats induced by high dose of vitamin A". The Journal of Veterinary Medical Science 62 (3): 293–9. March 2000. doi:10.1292/jvms.62.293. PMID 10770602.
- ↑ "Hypervitaminosis A-induced premature closure of epiphyses (physeal obliteration) in humans and calves (hyena disease): a historical review of the human and veterinary literature". Pediatric Radiology 37 (12): 1264–7. December 2007. doi:10.1007/s00247-007-0604-0. PMID 17909784.
- ↑ "Spontaneous fracture: multiple causes". The Consultant Pharmacist 24 (2): 100–2, 105–8, 110–2. February 2009. doi:10.4140/TCP.n.2009.100. PMID 19275452.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 21.13 21.14 21.15 "The acute and chronic toxic effects of vitamin A". The American Journal of Clinical Nutrition 83 (2): 191–201. February 2006. doi:10.1093/ajcn/83.2.191. PMID 16469975. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=16469975.
- ↑ "[Uremic pruritus]". Acta Medica Croatica 62 (Suppl 1): 32–6. 2008. PMID 18578330.
- ↑ "Severe hypervitaminosis A in siblings: evidence of variable tolerance to retinol intake". The Journal of Pediatrics 111 (4): 507–12. October 1987. doi:10.1016/s0022-3476(87)80109-9. PMID 3655980.
- ↑ 24.0 24.1 24.2 24.3 24.4 "Is vitamin A consumption a risk factor for osteoporotic fracture?". The Proceedings of the Nutrition Society 62 (4): 845–50. November 2003. doi:10.1079/PNS2003306. PMID 15018484.
- ↑ 25.0 25.1 25.2 "The vitamin A content and toxicity of bear and seal liver". The Biochemical Journal 37 (2): 166–8. July 1943. doi:10.1042/bj0370166. PMID 16747610.
- ↑ The Phoca barbata listed on pages 167–168 of the previous reference is now known as Erignathus barbatus
- ↑ "Hypervitaminosis A Following the Ingestion of Fish Liver: Report on 3 Cases from the Poison Control Center in Marseille". Wilderness Environ Med 31 (4): 454–456. December 2020. doi:10.1016/j.wem.2020.06.003. PMID 32861618.
- ↑ "Walrus, liver, raw (Alaska Native)". Mealographer. http://www.mealographer.com/food/Walrus/liver-raw-Alaska-Native-35083.html.
- ↑ "Moose, liver, braised (Alaska Native)". Mealographer. http://www.mealographer.com/food/Moose/liver-braised-Alaska-Native-35051.html.
- ↑ Myhre, Anne M; Monica H Carlsen; Siv K Bøhn; Heidi L Wold; Petter Laake; Rune Blomhoff (2003-12-01). "Water-Miscible, Emulsified, and Solid Forms of Retinol Supplements Are More Toxic Than Oil-Based Preparations". The American Journal of Clinical Nutrition 78 (6): 1152–1159. doi:10.1093/ajcn/78.6.1152. ISSN 0002-9165. PMID 14668278. http://www.ajcn.org/content/78/6/1152. Retrieved 2012-04-16.
- ↑ "The multifaceted nature of retinoid transport and metabolism". Hepatobiliary Surgery and Nutrition 3 (3): 126–39. June 2014. doi:10.3978/j.issn.2304-3881.2014.05.04. PMID 25019074.
- ↑ "Effects of hypervitaminosis A on the bone and mineral metabolism of the rat". Endocrinology 122 (6): 2933–9. June 1988. doi:10.1210/endo-122-6-2933. PMID 3371268.
- ↑ "Vitamin A and Retinoids as Mitochondrial Toxicants". Oxidative Medicine and Cellular Longevity 2015: 1–13. 2015. doi:10.1155/2015/140267. PMID 26078802.
- ↑ "Excessive dietary vitamin E: its alleviation of hypervitaminosis A and lack of toxicity". Poultry Science 49 (4): 1050–1. July 1970. doi:10.3382/ps.0491050. PMID 5485475.
- ↑ "Vitamin A toxicity: when one a day doesn't keep the doctor away". Liver Transplantation 12 (12): 1888–91. December 2006. doi:10.1002/lt.21007. PMID 17133567.
- ↑ "Activity of essential phospholipids (EPL) from soybean in liver diseases". Pharmacological Reports 63 (3): 643–59. 2011. doi:10.1016/S1734-1140(11)70576-X. PMID 21857075.
- ↑ "Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress". Journal of Hepatology 50 (6): 1236–46. June 2009. doi:10.1016/j.jhep.2009.01.025. PMID 19398233.
- ↑ "Hepatic stellate cells: a target for the treatment of liver fibrosis". Journal of Gastroenterology 35 (9): 665–72. 2000. doi:10.1007/s005350070045. PMID 11023037.
- ↑ "Dilinoleoylphosphatidylcholine is responsible for the beneficial effects of polyenylphosphatidylcholine on ethanol-induced mitochondrial injury in rats". Biochemical and Biophysical Research Communications 291 (4): 1109–12. March 2002. doi:10.1006/bbrc.2002.6557. PMID 11866479.
- ↑ "Hypervitaminosis A and fractures". The New England Journal of Medicine 348 (4): 347–9. January 2003. doi:10.1056/NEJMe020167. PMID 12540650.
- ↑ Nataraja, Anjali (2002). "Man's best friend?". Student BMJ 10: 131–70. http://student.bmj.com/student/view-article.html?id=sbmj0205158.
- ↑ "Mawson and Mertz: a re-evaluation of their ill-fated mapping journey during the 1911-1914 Australasian Antarctic Expedition". The Medical Journal of Australia 183 (11–12): 638–41. 2005. doi:10.5694/j.1326-5377.2005.tb00064.x. PMID 16336159. https://www.mja.com.au/journal/2005/183/11/mawson-and-mertz-re-evaluation-their-ill-fated-mapping-journey-during-1911-1914.
- ↑ "Accumulation of vitamin A in the hepatic stellate cell of arctic top predators". Anatomical Record 295 (10): 1660–8. October 2012. doi:10.1002/ar.22555. PMID 22907891.
- ↑ "Vitamin A toxicity and vitamin E deficiency in a rabbit colony". Contemporary Topics in Laboratory Animal Science 43 (4): 26–30. July 2004. PMID 15264766.
- ↑ "Vitamin E prevents side effects of high doses of vitamin A in chicks". Annals of the New York Academy of Sciences 669 (1): 396–8. September 1992. doi:10.1111/j.1749-6632.1992.tb17134.x. PMID 1444058. Bibcode: 1992NYASA.669..396W.
- ↑ Yeh, Yen-Hung; Lee, Ya-Ting; Hsieh, Hung-Sheng; Hwang, Deng-Fwu (2008). "Effect of taurine on toxicity of vitamin a in rats". Food Chemistry 106: 260–8. doi:10.1016/j.foodchem.2007.05.084.
- ↑ "Biliary metabolites of all-trans-retinoic acid in the rat". Archives of Biochemistry and Biophysics 224 (1): 13–8. July 1983. doi:10.1016/0003-9861(83)90185-6. PMID 6870249.
- ↑ "The biological activity of retinotaurine". The Journal of Nutrition 112 (8): 1626–30. August 1982. doi:10.1093/jn/112.8.1626. PMID 7097369. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=7097369.
- ↑ "Effect of cholestin on toxicity of vitamin A in rats". Food Chemistry 132 (1): 311–8. May 2012. doi:10.1016/j.foodchem.2011.10.082. PMID 26434295.
- ↑ "The action of vitamin K in hypervitaminosis A". The Biochemical Journal 41 (4): 575–80. 1947. doi:10.1042/bj0410575. PMID 16748217.
External links
| Classification | |
|---|---|
| External resources |
- Facts about Vitamin A and Carotenoids, from the National Institutes of Health's Office of Dietary Supplements.
 |


