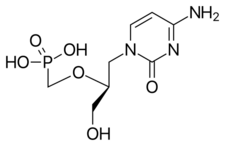
Chemistry:Cidofovir
 | |
| Clinical data | |
|---|---|
| Trade names | Vistide |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | complete |
| Protein binding | <6% |
| Elimination half-life | 2.6 hours (active metabolites: 15–65 hours) |
| Excretion | renal The above pharmacokinetic parameters are measured for cidofovir used in conjunction with probenecid.[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
| Formula | C8H14N3O6P |
| Molar mass | 279.189 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | -97.3 |
| Melting point | 260 °C (500 °F) |
| |
| |
| (verify) | |
Cidofovir, brand name Vistide, is a topical or injectable antiviral medication primarily used as a treatment for cytomegalovirus (CMV) retinitis (an infection of the retina of the eye) in people with AIDS.[2][3]
Cidofovir was approved for medical use in 1996.[4]
Medical use
DNA virus
Its only indication that has received regulatory approval worldwide is cytomegalovirus retinitis.[2][3] Cidofovir has also shown efficacy in the treatment of aciclovir-resistant HSV infections.[5] Cidofovir has also been investigated as a treatment for progressive multifocal leukoencephalopathy with successful case reports of its use.[6] Despite this, the drug failed to demonstrate any efficacy in controlled studies.[7] Cidofovir might have anti-smallpox efficacy and might be used on a limited basis in the event of a bioterror incident involving smallpox cases.[8] Brincidofovir, a cidofovir derivative with much higher activity against smallpox that can be taken orally has been developed.[9] It has inhibitory effects on varicella-zoster virus replication in vitro although no clinical trials have been done to date, likely due to the abundance of safer alternatives such as aciclovir.[10] Cidofovir shows anti-BK virus activity in a subgroup of transplant recipients.[11] Cidofovir is being investigated as a complementary intralesional therapy against papillomatosis caused by HPV.[12][13]
It first received FDA approval on 26 June 1996,[14] TGA approval on 30 April 1998[3] and EMA approval on 23 April 1997.[15]
It has been used topically to treat warts.[16]
Other
It has been suggested as an antitumour agent, due to its suppression of FGF2.[17][18][19]
Administration
Cidofovir is only available as an intravenous formulation. Cidofovir is to be administered with probenecid which decreases side effects to the kidney.[20] Probenecid mitigates nephrotoxicity by inhibiting organic anion transport of the proximal tubule epithelial cells of the kidney.[21] In addition, hydration must be administered to patients receiving cidofovir. 1 liter of normal saline is recommended in conjunction with each dose of cidofovir.[20]
Side effects
The major dose-limiting side effect of cidofovir is nephrotoxicity (i.e., kidney damage).[22] Other common side effects (occurring in >1% of people treated with the drug) include:[2][22]
- Nausea
- Vomiting
- Neutropenia
- Hair loss
- Weakness
- Headache
- Chills
- Decreased intraocular pressure
- Uveitis
- Iritis
Whereas uncommon side effects include: anaemia and elevated liver enzymes and rare side effects include: tachycardia and Fanconi syndrome.[22] Probenecid (a uricosuric drug) and intravenous saline should always be administered with each cidofovir infusion to prevent this nephrotoxicity.[23]
Contraindications
Hypersensitivity to cidofovir or probenecid (as probenecid needs to be given concurrently to avoid nephrotoxicity).[2]
Interactions
It is known to interact with nephrotoxic agents (e.g. amphotericin B, foscarnet, IV aminoglycosides, IV pentamide, vancomycin, tacrolimus, non-steroid anti-inflammatory drugs, etc.) to increase their nephrotoxic potential.[2][3] As it must be given concurrently with probenecid it is advised that drugs that are known to interact with probenecid (e.g. drugs that probenecid interferes with the renal tubular secretion of, such as paracetamol, aciclovir, aminosalicylic acid, etc.) are also withheld.[3]
Mechanism of action
Its active metabolite, cidofovir diphosphate, inhibits viral replication by selectively inhibiting viral DNA polymerases.[3] It also inhibits human polymerases, but this action is 8–600 times weaker than its actions on viral DNA polymerases.[3] It also incorporates itself into viral DNA, hence inhibiting viral DNA synthesis during reproduction.[3]
It possesses in vitro activity against the following viruses:[24]
- Human herpesviruses
- Adenoviruses
- Human poxviruses (including the smallpox virus)
- Human papillomavirus
History
Cidofovir was discovered at the Institute of Organic Chemistry and Biochemistry, Prague, by Antonín Holý, and developed by Gilead Sciences[25] and is marketed with the brand name Vistide by Gilead in the US, and by Pfizer elsewhere.
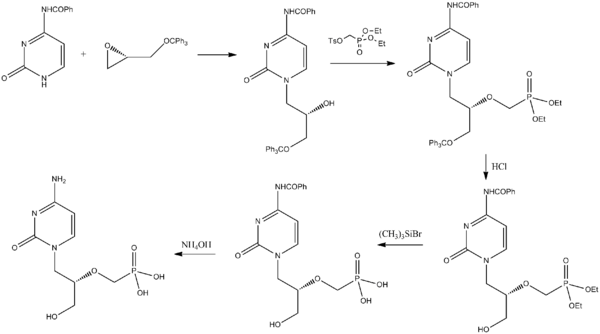
Synthesis
Cidofovir can be synthesized from a pyrimidone derivative and a protected derivative of glycidol.[26]
See also
- Brincidofovir, a novel prodrug of cidofovir that can be taken orally
References
- ↑ Cundy, Kenneth C. "Clinical Pharmacokinetics of the Antiviral Nucleotide Analogues Cidofovir and Adefovir." Clinical Pharmacokinetics 36.2 (1999): 127–143.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Vistide (cidofovir) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. http://reference.medscape.com/drug/vistide-cidofovir-342606#showall.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Product Information VISTIDE®". TGA eBusiness Services. Gilead Sciences Pty Ltd. 3 September 2013. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-01028-3.
- ↑ (in en) Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. 2012. p. 1502. ISBN 978-1437727029. https://books.google.com/books?id=nQ7-o8JAH7kC&pg=PA1502.
- ↑ "Management of acyclovir-resistant herpes simplex virus". Dermatologic Clinics 21 (2): 311–320. April 2003. doi:10.1016/S0733-8635(02)00093-1. PMID 12757254.
- ↑ "Use of cidofovir in progressive multifocal leukoencephalopathy". The Annals of Pharmacotherapy 35 (6): 741–744. June 2001. doi:10.1345/aph.10338. PMID 11408993.[yes|permanent dead link|dead link}}]
- ↑ "Human polyomavirus reactivation: disease pathogenesis and treatment approaches". Clinical & Developmental Immunology 2013: 373579. 2013. doi:10.1155/2013/373579. PMID 23737811.
- ↑ "Cidofovir in the treatment of poxvirus infections". Antiviral Research 55 (1): 1–13. July 2002. doi:10.1016/S0166-3542(02)00008-6. PMID 12076747.
- ↑ "Orally available cidofovir derivative active against smallpox". Lancet 359 (9311): 1041. March 2002. doi:10.1016/S0140-6736(02)08115-1. PMID 11937193.
- ↑ "Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate". Antimicrobial Agents and Chemotherapy 49 (8): 3153–3162. August 2005. doi:10.1128/AAC.49.8.3153-3162.2005. PMID 16048917.
- ↑ "Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy". Pediatric Transplantation 10 (1): 32–37. February 2006. doi:10.1111/j.1399-3046.2005.00391.x. PMID 16499584.
- ↑ "Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis". European Archives of Oto-Rhino-Laryngology 265 (8): 871–879. August 2008. doi:10.1007/s00405-008-0658-0. PMID 18458927.
- ↑ "Cidofovir: to use or not to use?". Current Opinion in Otolaryngology & Head and Neck Surgery 16 (1): 86–90. February 2008. doi:10.1097/MOO.0b013e3282f43408. PMID 18197029.
- ↑ "Cidofovir Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. https://www.drugs.com/monograph/cidofovir.html.
- ↑ "Vistide : EPAR – Product Information". European Medicines Agency. Gilead Sciences International Ltd.. 7 November 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000121/WC500052072.pdf.
- ↑ "Topical cidofovir for viral warts in children". Journal of the European Academy of Dermatology and Venereology 25 (12): 1487–1489. December 2011. doi:10.1111/j.1468-3083.2010.03961.x. PMID 21261749.
- ↑ "In vitro activity of potential anti-poxvirus agents". Antiviral Research 57 (1–2): 35–40. January 2003. doi:10.1016/s0166-3542(02)00198-5. PMID 12615301.
- ↑ "Cidofovir Activity against Poxvirus Infections". Viruses 2 (12): 2803–2830. December 2010. doi:10.3390/v2122803. PMID 21994641.
- ↑ "Interim Clinical Guidance for the Treatment of Monkeypox.". U.S. Centers for Disease Control and Prevention. 26 May 2022. https://www.cdc.gov/poxvirus/monkeypox/treatment.html.
- ↑ 20.0 20.1 "Details". http://www.gilead.com/~/media/Files/pdfs/medicines/other/vistide/vistide.pdf.
- ↑ "Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys". Toxicological Sciences 44 (2): 97–106. August 1998. doi:10.1006/toxs.1998.2481. PMID 9742650.
- ↑ 22.0 22.1 22.2 Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 978-0-9805790-9-3.
- ↑ "Vistide (cidofovir)". Gilead Sciences. September 2010. pp. Dosage and Administration: Dosage. http://www.gilead.com/~/media/Files/pdfs/medicines/other/vistide/vistide.pdf.
- ↑ "Clinical uses of cidofovir". Reviews in Medical Virology 7 (3): 145–156. September 1997. doi:10.1002/(SICI)1099-1654(199709)7:3<145::AID-RMV196>3.0.CO;2-0. PMID 10398479.
- ↑ "Press Releases: Gilead". http://www.gilead.com/pr_881577.
- ↑ "A practical synthesis of (S)-HPMPC". Tetrahedron Letters 35 (20): 3243. 1994. doi:10.1016/S0040-4039(00)76875-4.
 |